Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
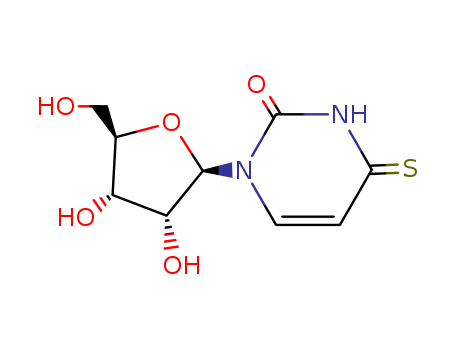
13957-31-8
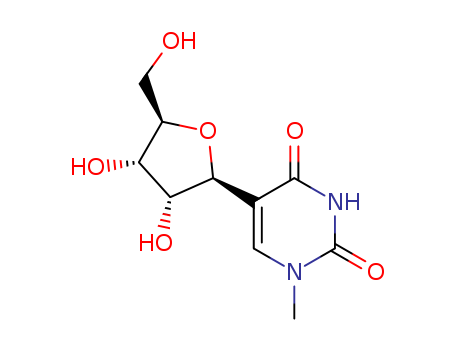
- Product Name:4-thiouridine
- Molecular Formula:C9H12N2O5S
- Purity:99%
- Molecular Weight:260.271
Product Details;
CasNo: 13957-31-8
Molecular Formula: C9H12N2O5S
Appearance: Light yellow crystalline
Description 4-Thiouridine (4-SU) is a photoactivatable ribonucleoside analog that is widely used for RNA analysis, including short-range RNA-RNA crosslinking and nascent RNA labeling. The crosslinking thio moiety is attached directly to the nucleotide base, thus 4-SU differs from uridine only by a single sulfur substitution. This offers the advantage of incorporating into an RNA chain with minimal structural perturbation and with similar base-pairing properties, reducing the likelihood that substitution will impair RNA interactions or activities. Uses Nucleotide analogue, essential for cell growth in certain bacterial species. This compound is also able to chelate with certain metal ions, and in tRNA it can act as a built-in antiphotomutagenic agent that protects Escherichia coli cells against mutagenesis. Definition ChEBI: A thiouridine in which the oxygen replaced by sulfur is that at C-4. General Description 4-Thiouridine is a photoreactive uridine analog. Biochem/physiol Actions 4-Thiouridine plays a role in assessing nascent RNA synthesis. It also enhances site-specific crosslinking. 4-Thiouridine also acts as a photoaffinity probe due to its stability even while lacking specific photoactivation. It can also be incorporated into RNAs. InChI:InChI=1/C9H12N2O5S/c12-3-4-6(13)7(14)8(16-4)11-2-1-5(17)10-9(11)15/h1-2,4,6-8,12-14H,3H2,(H,10,15,17)
-
In an aqueous solution of sodium hydrosu...
-
Ribonucleic acid (RNA) is central to man...
The invention provides an anti-hepatitis...
This invention provides expressible poly...
Unambiguous characterization of 5-substi...
4-thio-1-(2',3',5'-tri-O-acetyl-β-D-ribofuranosyl)-(3H)-pyrimidine-2,4-dione 4-thiouridine uridine 4-thiouridine
1-(2',3',5'-tri-O-benzoyl-β-D-ribofuranosyl)-4-thiouracil
2-methoxy-1-β-D-ribofuranosyl-pyrimidine-4-one
S-(2-cyanoethyl)-4-thiouridine
4-ethoxy-1-(β-D-ribofuranosyl)-2(1H)-pyrimidinone
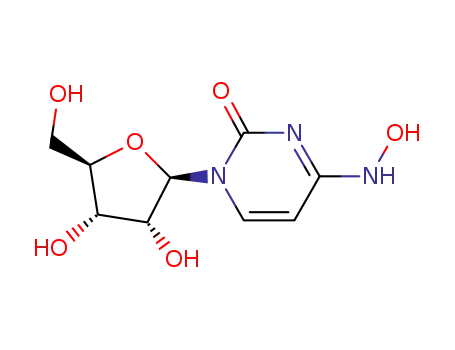
1-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-4-(hydroxylamino)pyrimidin-2(1H)-one
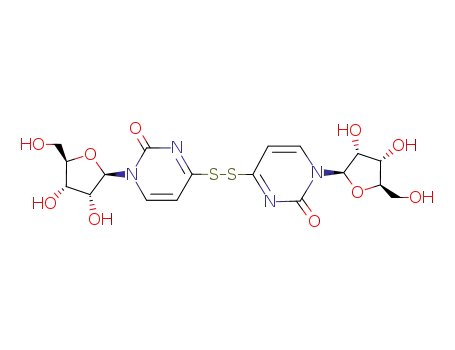
Di-(4-deoxy-4-uridinyl)-disulfid
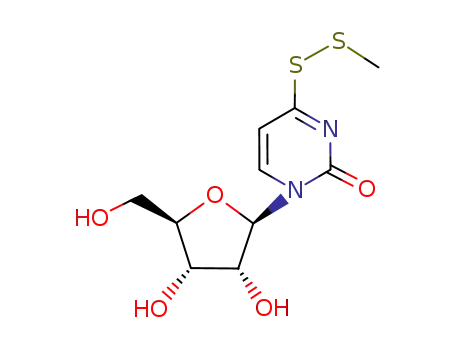
1-((2R,3R,4S,5R)-3,4-Dihydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-4-methyldisulfanyl-1H-pyrimidin-2-one
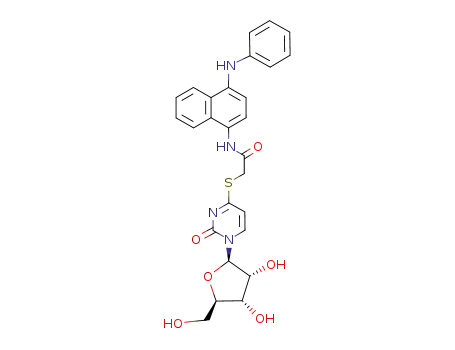
S-(N-(4-anilino-1-naphthyl)carbamylmethyl)-4-thiouridine
High Quality Factory Sells 4-thiouridine 13957-31-8 In Bulk Supply
4-THIOURIDINE(Cas 13957-31-8) Usage
13957-31-8 Relevant articles
Isolation and characterization of pyrimidine sulfenic acid via scission of the sulfur-sulfur bond in the methyl analog of bis(4-thiouridine) disulfide.
Pal,Uziel,Doherty,Cohn
, p. 3634 - 3638 (1969)
Chemical Conversion of Uridine into 4-Thiouridine via the 4-(1,2,4-Triazol-1-yl)pyrimidin-2(1H)-one Intermediate
Sung, Wing L.
, p. 522 - 523 (1982)
Some synthetic analogues of uridine diphosphate glucose.
Kochetkov,Budowsky,Shibaev,Yeliseeva,Grachev,Demushkin
, p. 1207 - 1218 (1963)
Synthesis and Metabolic Fate of 4-Methylthiouridine in Bacterial tRNA
Borek, Christoph,Reichle, Valentin F.,Kellner, Stefanie
, p. 2768 - 2771 (2020)
Anti-hepatitis B virus compound as well as preparation method and application thereof
-
Paragraph 0033-0035, (2021/06/22)
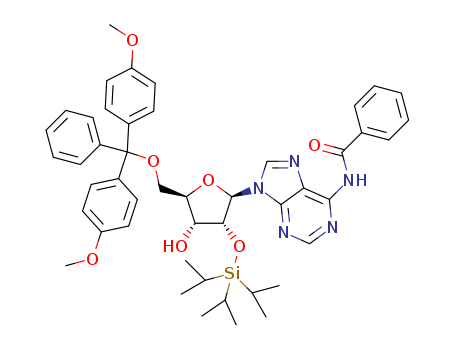
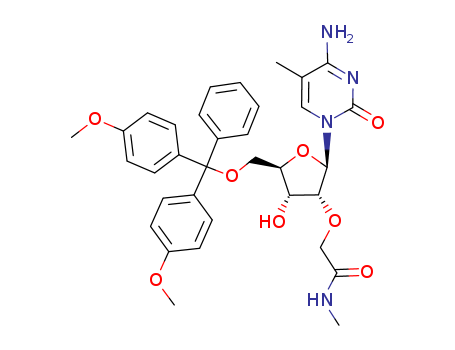
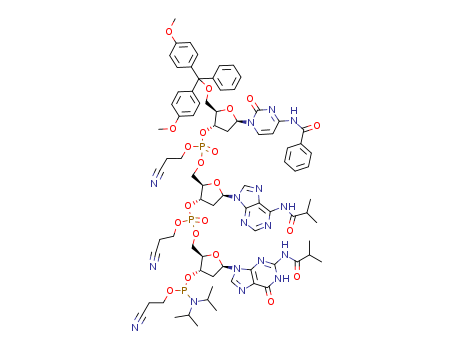
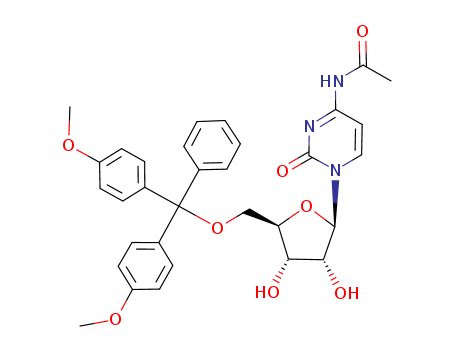
SYNTHESIS AND STRUCTURE OF HIGH POTENCY RNA THERAPEUTICS
-
, (2019/01/15)
Systematic assignment of NMR spectra of 5-substituted-4-thiopyrimidine nucleosides
Zhang, Xiaohui,Wang, Jian,Xu, Yao-Zhong
, p. 523 - 529 (2013/09/02)
13957-31-8 Process route
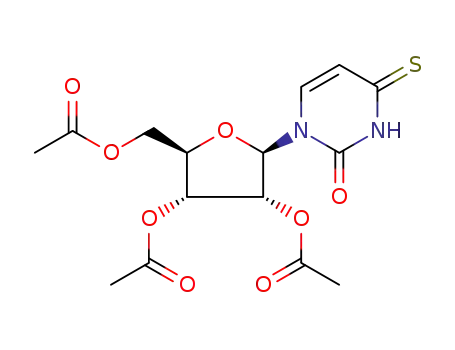

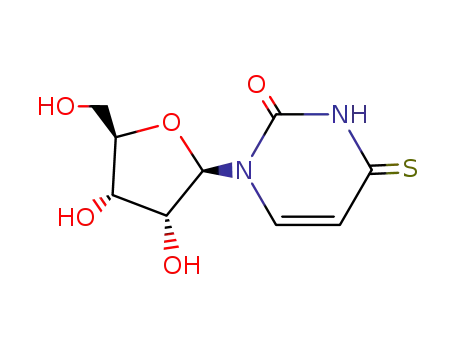
Conditions
Yield
90%
89%
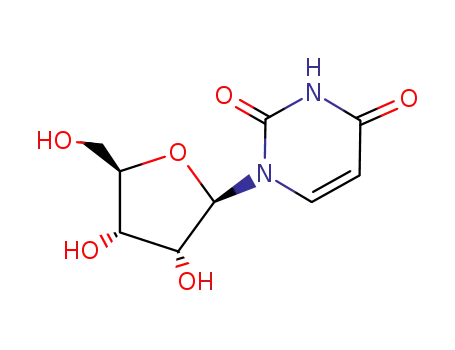


Conditions
Yield
13957-31-8 Upstream products
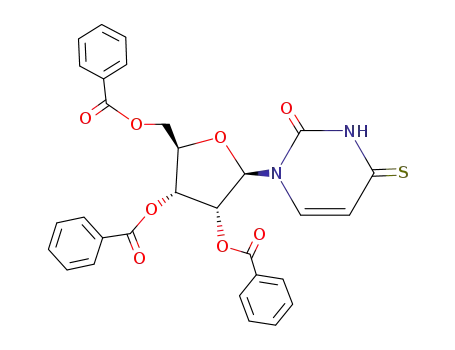
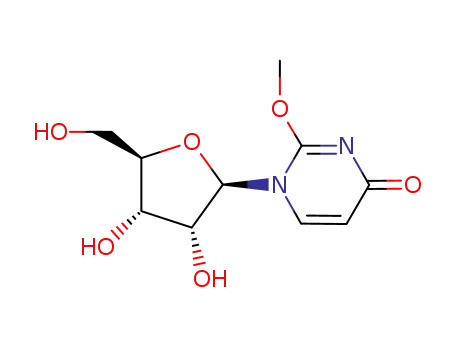
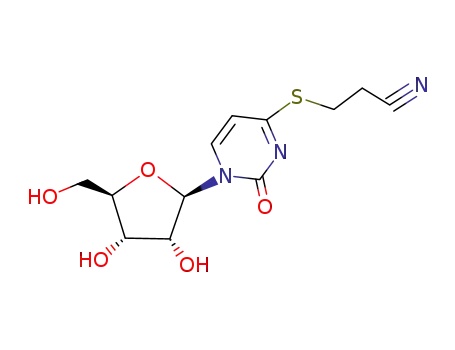
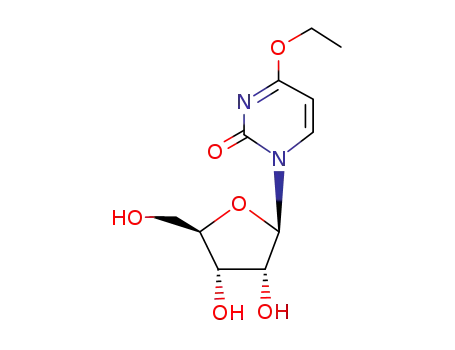
13957-31-8 Downstream products