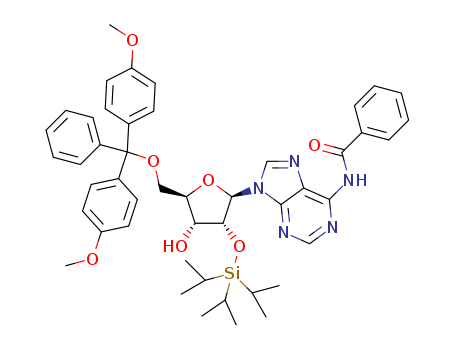
Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
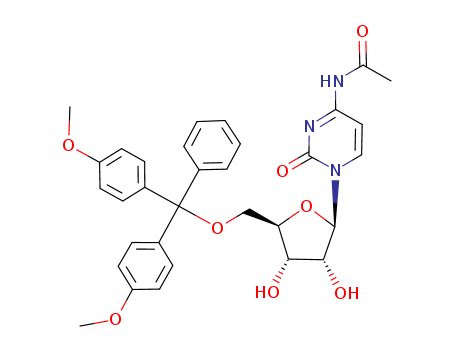
121058-82-0
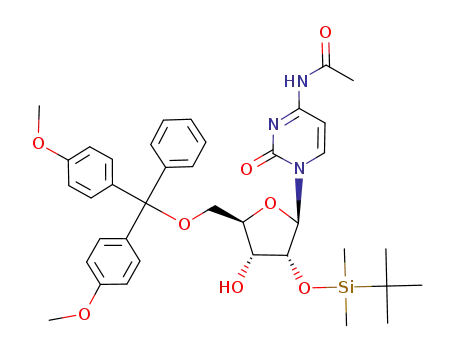
- Product Name:5'-DMT-Ac-rC
- Molecular Formula:C32H33 N3 O8
- Purity:99%
- Molecular Weight:587.629
Product Details;
CasNo: 121058-82-0
Molecular Formula: C32H33 N3 O8
Use of methylamine or methylamine/ammoni...
UNA (unlocked nucleic acid) monomers are...
A base-labile group for 2'-OH protection...
(Chemical Equation Presented) A novel me...
A new class of glycosyltransferase inhib...
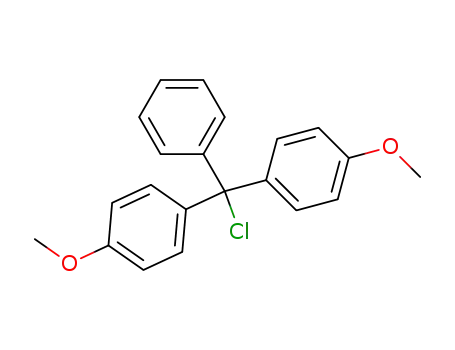
4,4'-dimethoxytrityl chloride N-acetylcytidine N4-acetyl-5'-O-(4,4'-dimethoxytrityl)cytidine 4,4'-dimethoxytrityl chloride acetic anhydride CYTIDINE N4-acetyl-5'-O-(4,4'-dimethoxytrityl)cytidine
4,4'-dimethoxytrityl chloride
N-acetylcytidine
acetic anhydride
CYTIDINE
5'-O-DMTr-2'-O-TBDMS-N4-acetylcytidine
N-{1-[(2R,3R,4S,5R)-5-[Bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-4-(tert-butyl-dimethyl-silanyloxy)-3-hydroxy-tetrahydro-furan-2-yl]-2-oxo-1,2-dihydro-pyrimidin-4-yl}-acetamide
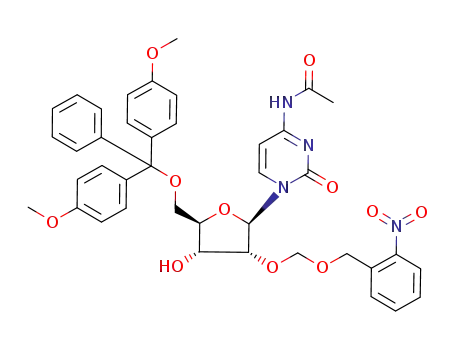
N-{1-[(2R,3R,4R,5R)-5-[Bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-4-hydroxy-3-(2-nitro-benzyloxymethoxy)-tetrahydro-furan-2-yl]-2-oxo-1,2-dihydro-pyrimidin-4-yl}-acetamide
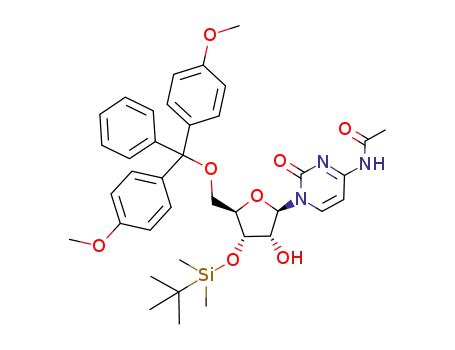
N4-acetyl-5'-O-(4,4'-dimethoxytrityl)-2'-O-{[(triisopropylsilyl)oxy]methyl}cytidine
High Quality For Sale 5'-DMT-Ac-rC 121058-82-0 In Stock
121058-82-0 Relevant articles
Methylamine Deprotection Provides Increased Yield of Oligoribonucleotides
Reddy, M. P.,Farooqui, Firdous,Hanna, Naeem B.
, p. 8929 - 8932 (1995)
UNA (unlocked nucleic acid): A flexible RNA mimic that allows engineering of nucleic acid duplex stability
Langkjaer, Niels,Pasternak, Anna,Wengel, Jesper
experimental part, p. 5420 - 5425 (2009/12/06)
A base-labile group for 2′-OH protection of ribonucleosides: A major challenge for RNA synthesis
Lavergne, Thomas,Bertrand, Jean-Remi,Vasseur, Jean-Jacques,Debart, Francoise
scheme or table, p. 9135 - 9138 (2009/10/01)
A new RNA synthetic method with a 2′-O-(2-cyanoethoxymethyl) protecting group
Ohgi, Tadaaki,Masutomi, Yutaka,Ishiyama, Kouichi,Kitagawa, Hidetoshi,Shiba, Yoshinobu,Yano, Junichi
, p. 3477 - 3480 (2007/10/03)
Synthesis of a new transition-state analog of the sialyl donor. Inhibition of sialyltransferases
Sun, Hongbin,Yang, Jingsong,Amaral, Katie E.,Horenstein, Benjamin A.
, p. 2451 - 2453 (2007/10/03)
121058-82-0 Process route

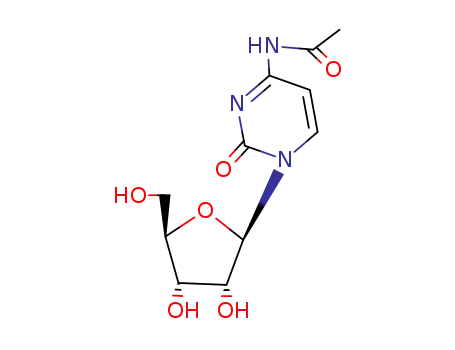

Conditions
Yield
65%
61%


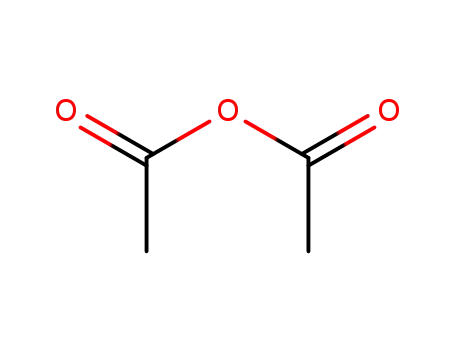

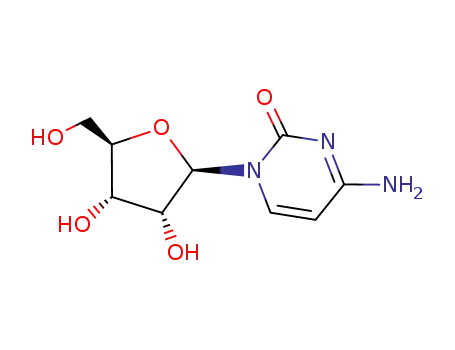

Conditions
Yield
70%
121058-82-0 Upstream products




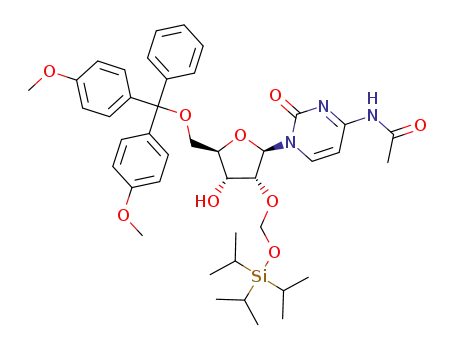
121058-82-0 Downstream products