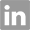Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
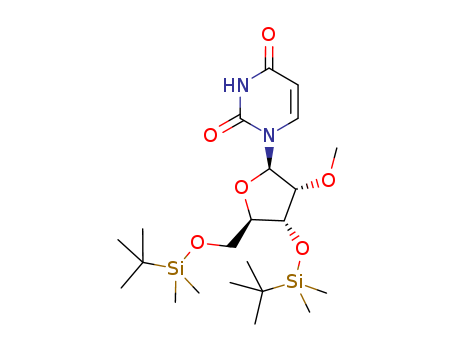
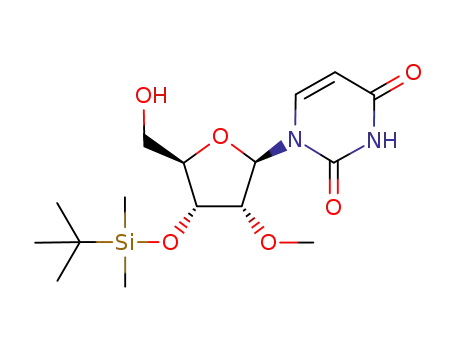
367511-37-3
- Product Name:1-((2R,3R,4R,5R)-4-((tert-Butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl)oxy)methyl)-3-methoxytetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione
- Molecular Formula:
- Purity:99%
- Molecular Weight:486.756
Product Details;
CasNo: 367511-37-3
The inherentin vivoinstability of oligon...
Various embodiments provide STOPS? polym...
The present disclosure provides alternat...
The present disclosure provides alternat...
tert-butyldimethylsilyl chloride 2'-O-methyluridine C22H42N2O6Si2 C22H42N2O6Si2 2’-O-(tert-butyldimethylsilyl)-5’-O-(4,4’-dimethoxytrityl)uridin-3’-yl 5’-O-levulinoyl-2’-O-methyluridin-3’-yl N,N-diisopropylphosphoramidite
tert-butyldimethylsilyl chloride
2'-O-methyluridine
1-((2R,3R,4R,5R)-4-((tert-butyldimethylsilyl)oxy)-5-(hydroxymethyl)-3-methoxytetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione
C24H43N5O5Si2
C23H45N3O5Si2
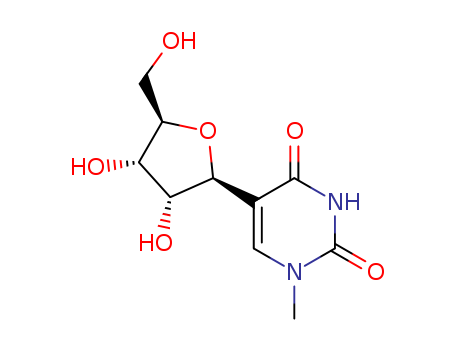
N4,2'-O-dimethylcytidine
High Purity Fast Delivery 1-((2R,3R,4R,5R)-4-((tert-Butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl)oxy)methyl)-3-methoxytetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione 367511-37-3 In Stock
367511-37-3 Relevant articles
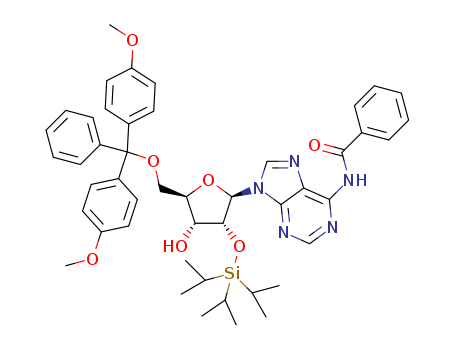
Synthesis of a novel cyclopropyl phosphonate nucleotide as a phosphate mimic
Altenhofer, Erich F.,Fowler-Watters, Matthew,Joyce, Leo A.,Kumar, Pankaj,Lawler, Michael J.,Li, Zhen,Pei, Tao
supporting information, p. 6808 - 6811 (2021/07/13)
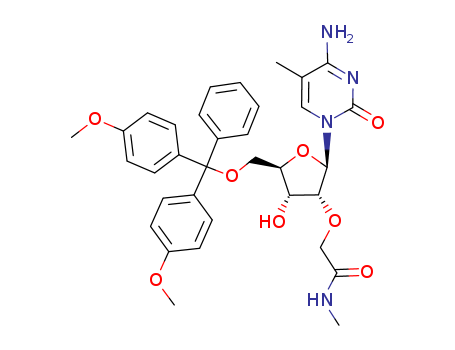
S-ANTIGEN TRANSPORT INHIBITING OLIGONUCLEOTIDE POLYMERS AND METHODS
-
Paragraph 0068; 0474, (2021/06/22)
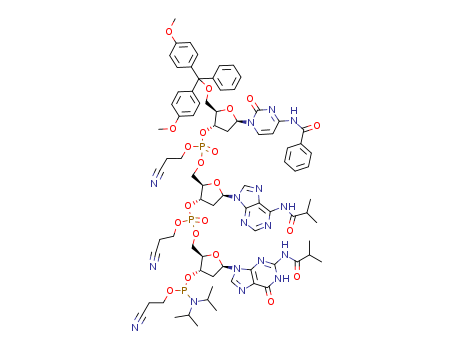
ALTERNATIVE NUCLEIC ACID MOLECULES AND USES THEREOF
-
Page/Page column 623, (2016/06/15)
ALTERNATIVE NUCLEIC ACID MOLECULES AND USES THEREOF
-
Page/Page column 631, (2016/06/28)
367511-37-3 Process route
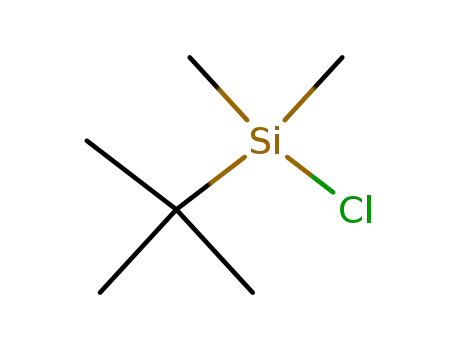

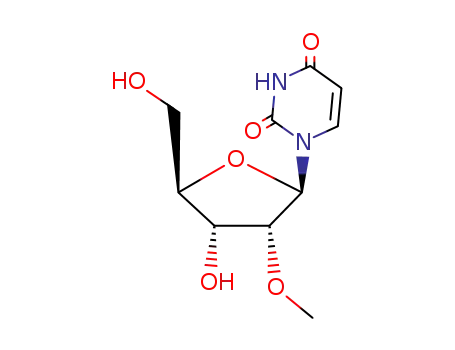

Conditions
Yield
85%
85%
85%
85%

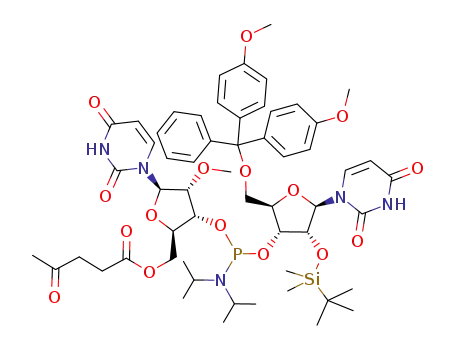
Conditions
Yield
367511-37-3 Upstream products
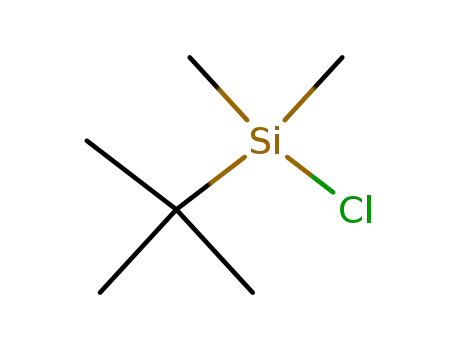

367511-37-3 Downstream products