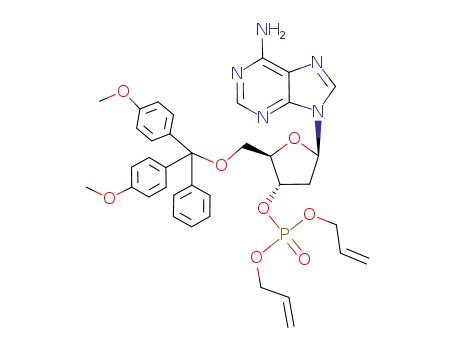
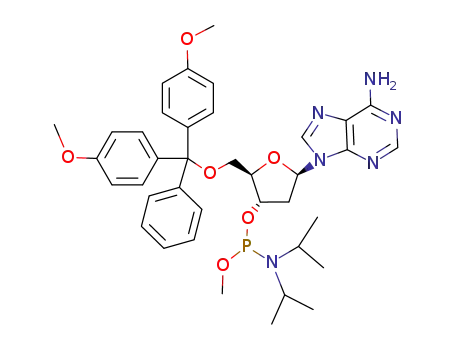
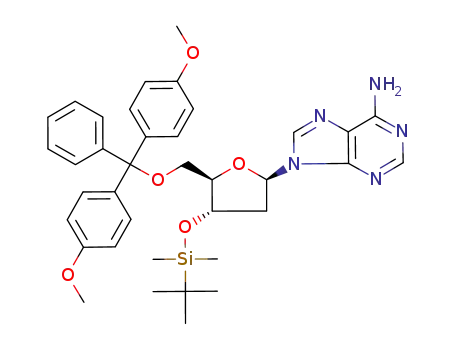
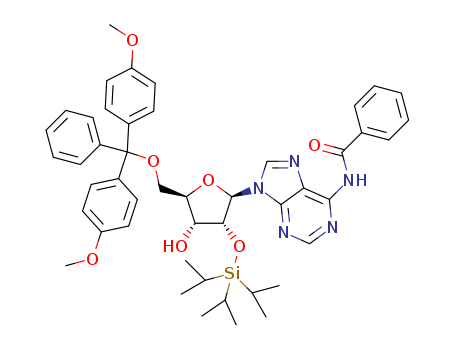
Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
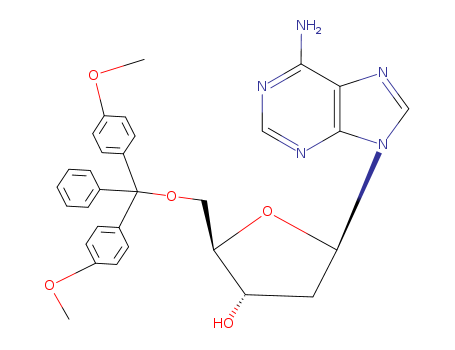
17331-22-5
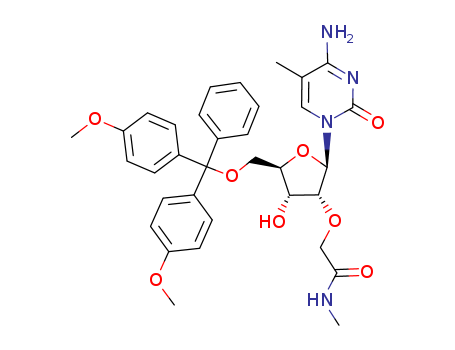
- Product Name:5'-O-(4,4'-DIMETHOXYTRITYL)-2'-DEOXYADENOSINE
- Molecular Formula:C31H31 N5 O5
- Purity:99%
- Molecular Weight:553.618
Product Details;
CasNo: 17331-22-5
Molecular Formula: C31H31 N5 O5
Application of the concept of transient ...
-
Phosphorothioate oligonucleotides manufa...
A modified nucleotide, intended for the ...
The acetyl capping reaction used through...
Disaccharide nucleosides constitute an i...
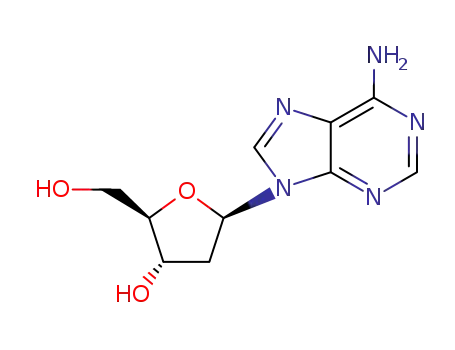
4,4'-dimethoxytrityl chloride 2'-deoxy-D-adenosine 5'-O-[bis(4-methoxyphenyl)phenylmethyl]-2'-deoxyadenosine 2-azidomethyl-N-(9-{5-[bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-4-hydroxy-tetrahydro-furan-2-yl}-9H-purin-6-yl)-benzamide 5'-O-[bis(4-methoxyphenyl)phenylmethyl]-2'-deoxyadenosine
4,4'-dimethoxytrityl chloride
2'-deoxy-D-adenosine
(9-{(2R,4S,5R)-5-[Bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-4-hydroxy-tetrahydro-furan-2-yl}-9H-purin-6-yl)-carbamic acid 2-(5-dimethylamino-naphthalene-1-sulfonyl)-ethyl ester
2'-deoxy-5'-O-(4,4'-dimethoxytrityl)-N6-phthaloyladenosine
Phosphoric acid diallyl ester (2R,3S,5R)-5-(6-amino-purin-9-yl)-2-[bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-tetrahydro-furan-3-yl ester
Diisopropyl-phosphoramidous acid (2R,3S,5R)-5-(6-amino-purin-9-yl)-2-[bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-tetrahydro-furan-3-yl ester methyl ester
5'-O-(4,4'-dimethoxytrityl)-2'-O-(tert-butyldimethylsilyl)-2'-deoxyadenosine
2'-deoxy-D-adenosine
Good Price Factory Sells High Purity 5'-O-(4,4'-DIMETHOXYTRITYL)-2'-DEOXYADENOSINE 17331-22-5
17331-22-5 Relevant articles
Transient Protection: Efficient One-Flask Syntheses of Protected Deoxynucleosides
Ti, G. S.,Gaffney, B. L.,Jones, R. A.
, p. 1316 - 1319 (1982)
A Convenient Method for the Synthesis of N-Free 5′-O-(p,p′-Dimethoxytrityl)-2′-deoxyribonucleosides via the 5′-O-Selective Tritylation of the Parent Substances
Kataoka, Masanori,Hayakawa, Yoshihiro
, p. 6087 - 6089 (1999)
Characterization of high molecular weight impurities in synthetic phosphorothioate oligonucleotides
Kurata, Christine,Bradley, Kym,Gaus, Hans,Luu, Nhuy,Cedillo, Isaiah,Ravikumar, Vasulinga T.,Sooy, Kent Van,McArdle, James V.,Capaldi, Daniel C.
, p. 607 - 614 (2006)
MODIFIED NUCLEOTIDES FOR SYNTHESIS OF NUCLEIC ACIDS, A KIT CONTAINING SUCH NUCLEOTIDES AND THEIR USE FOR THE PRODUCTION OF SYNTHETIC NUCLEIC ACID SEQUENCES OR GENES
-
Paragraph 0118, (2020/08/05)
Conversion of adenine to 5-amino-4-pyrimidinylimidazole caused by acetyl capping during solid phase oligonucleotide synthesis
Rodriguez, Andrew A.,Cedillo, Isaiah,McPherson, Andrew K.
, p. 3468 - 3471 (2016/07/21)
Synthesis of disaccharide nucleosides by the O-glycosylation of natural nucleosides with thioglycoside donors
Aoki, Shin,Fukumoto, Taketo,Itoh, Taiki,Kurihara, Masayuki,Saito, Shigeto,Komabiki, Shin-Ya
, p. 740 - 751 (2015/06/02)
17331-22-5 Process route



![5'-O-[bis(4-methoxyphenyl)phenylmethyl]-2'-deoxyadenosine](/upload/2023/7\bba23811-0dbf-45c6-bd1a-5b28b375b40d.png)
Conditions
Yield
86%
77%
68%

![5'-O-[bis(4-methoxyphenyl)phenylmethyl]-2'-deoxyadenosine](/upload/2023/7\bba23811-0dbf-45c6-bd1a-5b28b375b40d.png)
Conditions
Yield
90%
17331-22-5 Upstream products


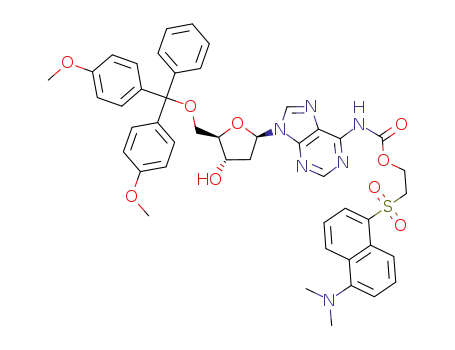
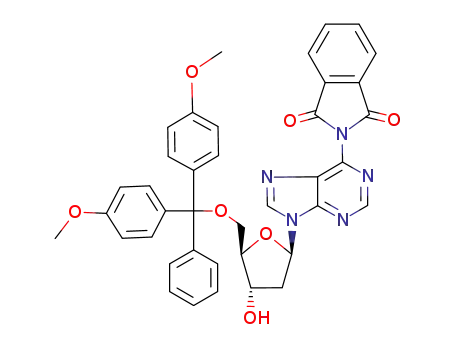
17331-22-5 Downstream products