Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
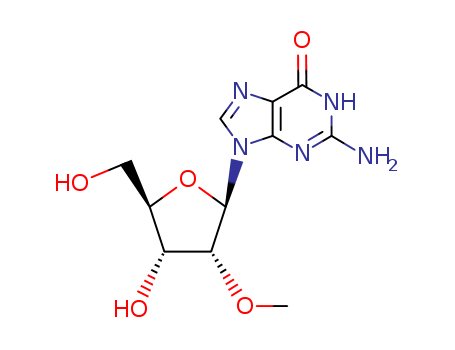
2140-71-8
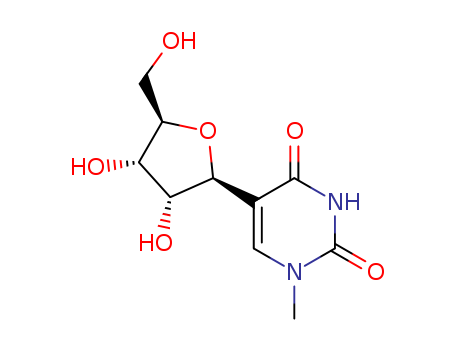
- Product Name:2'-O-Methylguanosine
- Molecular Formula:C11H15N5O5
- Purity:99%
- Molecular Weight:297.27
Product Details;
CasNo: 2140-71-8
Molecular Formula: C11H15N5O5
Appearance: White solid
The RNA-world hypothesis assumes that li...
The present invention provides an improv...
An improved strategy for the selective s...
An improved strategy for the synthesis o...
trimethylsulphonium hydroxide G 3’-O-methylguanosine 2'-O-methylguanosine 1,2'-Dimethylguanosine 1-methylguanosine trimethylsulphonium hydroxide G 3’-O-methylguanosine 2'-O-methylguanosine 1,3'-Dimethylguanosine 1-methylguanosine
2-amino-2'-O-methyladenosine
trimethylsulphonium hydroxide
G
6-(tert-Butyl-diphenyl-silanyloxy)-9-((2R,3R,3aR,9aR)-5,5,7,7-tetraisopropyl-3-methoxy-tetrahydro-1,4,6,8-tetraoxa-5,7-disila-cyclopentacycloocten-2-yl)-9H-purin-2-ylamine
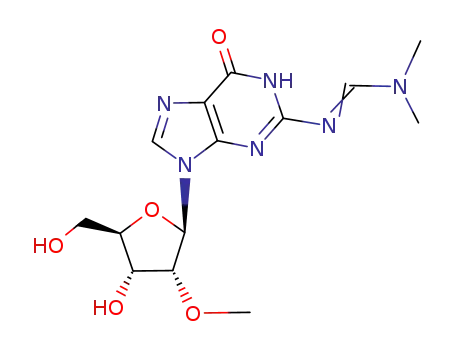
N2-dimethylaminomethylene-2'-O-methylguanosine
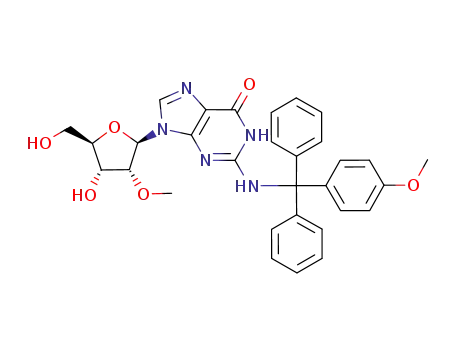
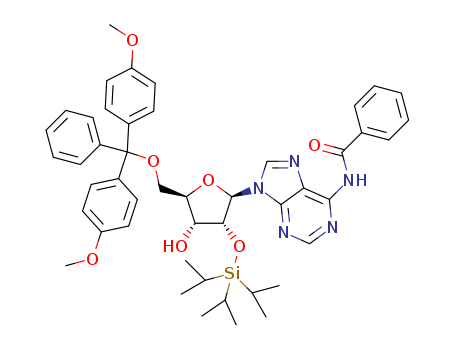
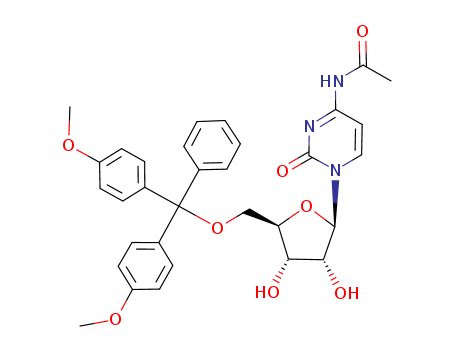
N2-(4-monomethoxytrityl)-2'-O-methylguanosine
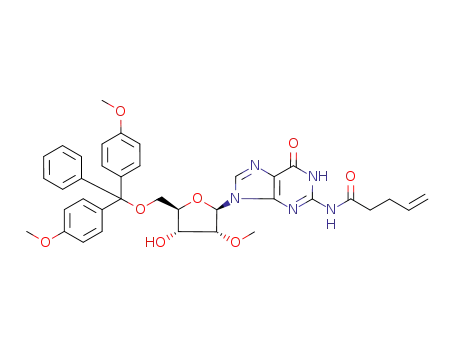
Pent-4-enoic acid (9-{(2R,3R,4R,5R)-5-[bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-4-hydroxy-3-methoxy-tetrahydro-furan-2-yl}-6-oxo-6,9-dihydro-1H-purin-2-yl)-amide
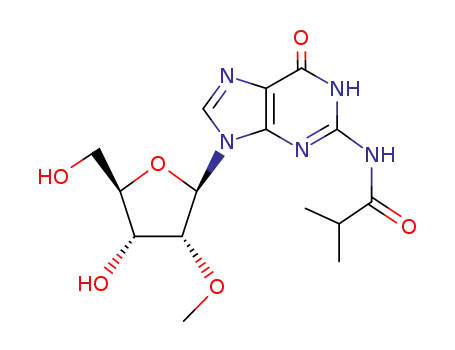
N2-isobutyryl-2′-O-methylguanosine
Fast Delivery Pharmaceutical Grade 2'-O-Methylguanosine 2140-71-8 In Stock
2140-71-8 Relevant articles
Noncanonical RNA Nucleosides as Molecular Fossils of an Early Earth—Generation by Prebiotic Methylations and Carbamoylations
Schneider, Christina,Becker, Sidney,Okamura, Hidenori,Crisp, Antony,Amatov, Tynchtyk,Stadlmeier, Michael,Carell, Thomas
supporting information, p. 5943 - 5946 (2018/04/30)
PROCESS FOR THE SYNTHESIS OF 2'-O-SUBSTITUTED PURINES
-
Page/Page column 8, (2008/12/07)
An efficient process for synthesis of 2′-O-methyl and 3′-O-methyl guanosine from 2-aminoadenosine using diazomethane and the catalyst stannous chloride
Kore, Anilkumar,Parmar, Gaurang,Reddy, Srinu
, p. 307 - 314 (2007/10/03)
MDPSCL2: a new protecting group for chemoselective synthesis of 2'-O-alkylated guanosines.
Chow, Suetying,Wen,Sanghvi, Yogesh S,Theodorakis, Emmanuel A
, p. 583 - 587 (2007/10/03)
2140-71-8 Process route
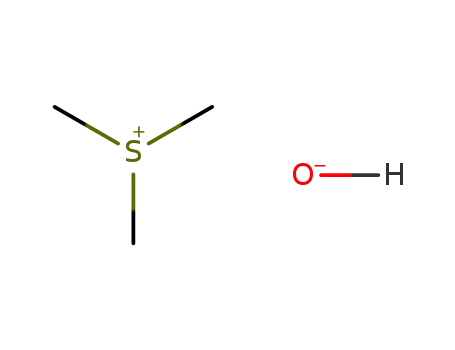

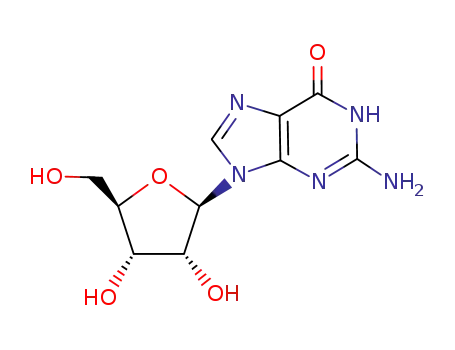

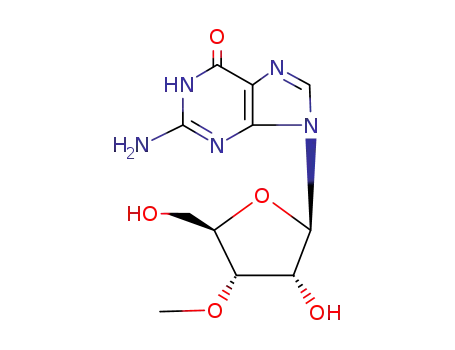

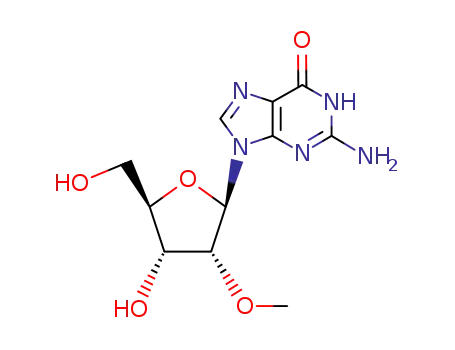

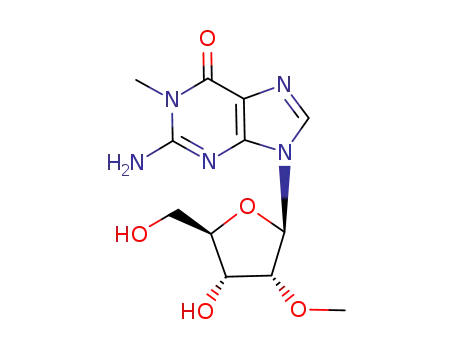

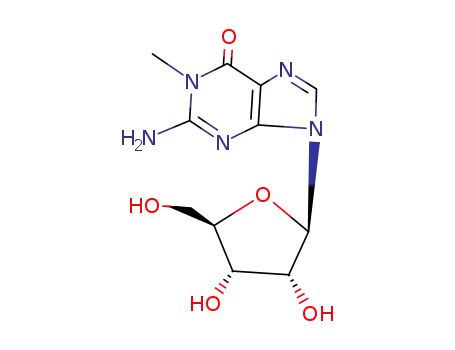
Conditions
Yield
11 % Spectr.
4 % Spectr.
23 % Spectr.
53%
18%
17%
15%
16%
18%
15%
15%
17%
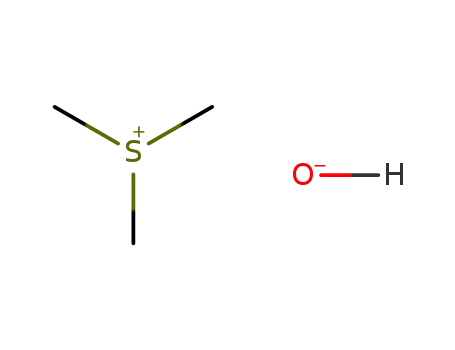







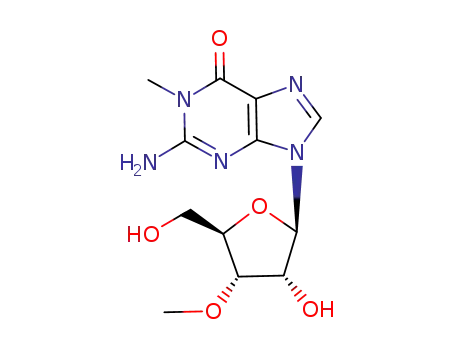


Conditions
Yield
18%
17%
16%
12%
2140-71-8 Upstream products
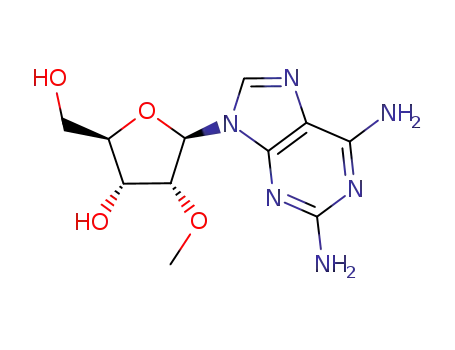
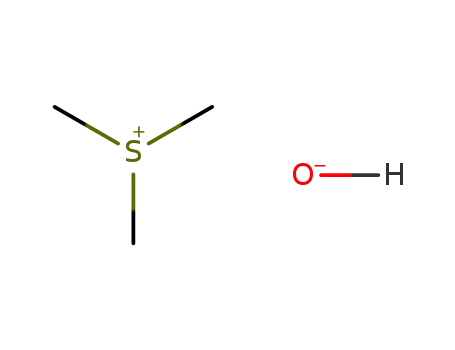

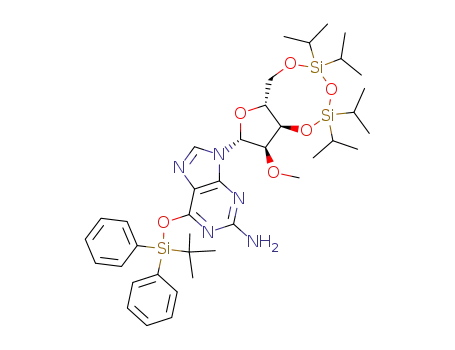
2140-71-8 Downstream products