Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
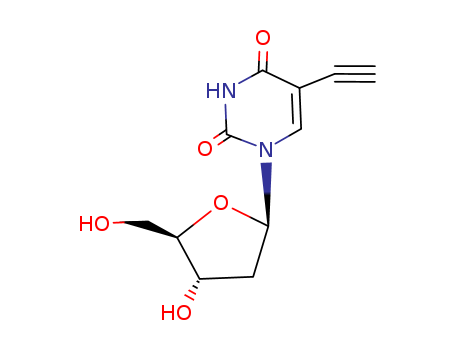
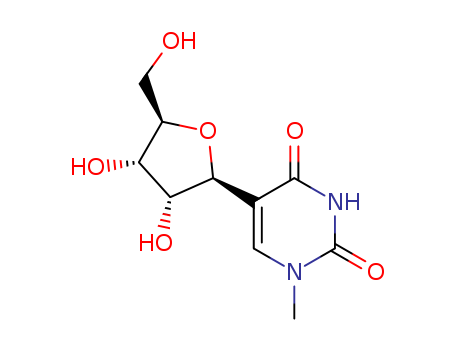
61135-33-9
- Product Name:5-ETHYNYL-2'-DEOXYURIDINE
- Molecular Formula:C11H12N2O5
- Purity:99%
- Molecular Weight:252.227
Product Details;
CasNo: 61135-33-9
Molecular Formula: C11H12N2O5
Description 5-Ethynyl-2′-deoxyuridine (61135-33-9) is a novel agent for fast and sensitive detection of DNA synthesis?in vivo.1 5-EdU is incorporated into proliferating cells followed by detection with a fluorescent azide using click ligation.1 In contrast to the use of BrdU, the new method does not require sample fixation or DNA denaturation. 5-Ethynyl-2′-deoxyuridine may be used for studying cell proliferation in the central nervous system and may be combined with BrdU staining in a novel protocol allowing for double labeling of DNA synthesis.2 Uses 5-Ethynyl-2’deoxyuridine is a extensively used nucleoside analog used as a probe for DNA synthesis. However, 5-Ethynyl-2’deoxyuridine is a toxic anti-metabolite, which potentially causes DNA instabi
liy, necrosis and cell-cycle arrest, therefore it is often used for short-term DNA synthesis in tissue culture and living organisms where prolonged cell survival is not required. InChI:InChI=1/C11H12N2O5/c1-2-6-4-13(11(17)12-10(6)16)9-3-7(15)8(5-14)18-9/h1,4,7-9,14-15H,3,5H2,(H,12,16,17)/t7-,8+,9+/m0/s1
The representative DNA-labeling agent 5-...
Copper(I)-catalyzed azide–alkyne cycload...
Herpes simplex virus thymidine kinase ty...
A group of arabinouridines (TMSEAU, EAU,...
Tropolone (2-hydroxycyclohepta-2,4,6-tri...
Fluorescent supramolecular nucleoside-ba...
Nucleoside analogs represent a class of ...
We designed and synthesized several fluo...
5-ethynyltrimethylsilyl-3',5'-di-O-p-toluyl-2'-deoxyuridine 2'-deoxy-5-ethynyluridine 5-(1-chlorovinyl)-2'-deoxy-3',5'-di-O-(p-toluoyl)uridine 2'-deoxy-5-ethynyl-5'-O-(p-toluoyl)uridine 2'-deoxy-5-ethynyluridine
5-ethynyltrimethylsilyl-3',5'-di-O-p-toluyl-2'-deoxyuridine
5-(1-chlorovinyl)-2'-deoxy-3',5'-di-O-(p-toluoyl)uridine
1-(2-deoxy-β-D-ribofuranosyl)-5-[2-(trimethylsilyl)ethynyl]uracil
5-Iodo-2'-deoxyuridine
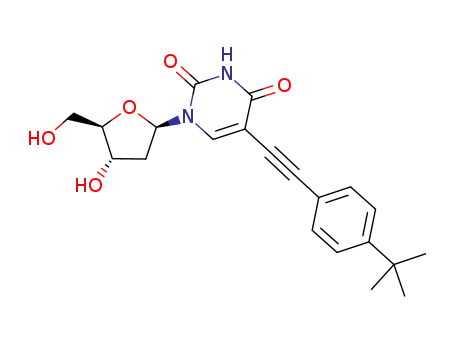
5-[(4-tert-butylphenyl)ethynyl]-2'-deoxyuridine
Good Producer Pharmaceutical Intermediates Best Price 5-ETHYNYL-2'-DEOXYURIDINE 61135-33-9
5-ETHYNYL-2'-DEOXYURIDINE(Cas 61135-33-9) Usage
61135-33-9 Relevant articles
Phosphorylated 5-ethynyl-2′-deoxyuridine for advanced DNA labeling
Seo, Siyoong,Onizuka, Kazumitsu,Nishioka, Chieko,Takahashi, Eiki,Tsuneda, Satoshi,Abe, Hiroshi,Ito, Yoshihiro
, p. 4589 - 4595 (2015)
Turning Off Transcription with Bacterial RNA Polymerase through CuAAC Click Reactions of DNA Containing 5-Ethynyluracil
Slaví?ková, Michaela,Janou?ková, Martina,?imonová, Anna,Cahová, Hana,Kambová, Milada,?anderová, Hana,Krásny, Libor,Hocek, Michal
, p. 8311 - 8314 (2018)
Synthesis of tricarbonyl rhenium and technetium complexes of a 5′-carboxamide 5-ethyl-2′-deoxyuridine for selective inhibition of herpes simplex virus thymidine kinase 1
Desbouis,Schubiger,Schibli
, p. 1340 - 1347 (2007)
5-Alkynyl analogs of arabinouridine and 2′-deoxyuridine: Cytostatic activity against herpes simplex virus and varicella-zoster thymidine kinase gene-transfected cells
Cristofoli, Walter A.,Wiebe, Leonard I.,De Clercq, Erik,Andrei, Graciela,Snoeck, Robert,Balzarini, Jan,Knaus, Edward E.
, p. 2851 - 2857 (2007)
Tropolone-Conjugated DNA: Fluorescence Enhancement in the Duplex
Bollu, Amarnath,Sharma, Nagendra K.
, p. 1467 - 1475 (2019)
Intermolecular hydrogen-bond interaction to promote thermoreversible 2'-deoxyuridine-based AIE-organogels
Zhao, Xuan,Zhao, Long,Xiao, Qiuyun,Xiong, Hai
supporting information, p. 1363 - 1367 (2020/10/27)
Thermodynamic Reaction Control of Nucleoside Phosphorolysis
Kaspar, Felix,Giessmann, Robert T.,Neubauer, Peter,Wagner, Anke,Gimpel, Matthias
supporting information, p. 867 - 876 (2020/01/24)
Direct incorporation and extension of a fluorescent nucleotide through rolling circle DNA amplification for the detection of microRNA 24-3P
Le, Binh Huy,Seo, Young Jun
supporting information, p. 2035 - 2038 (2018/05/04)
61135-33-9 Process route
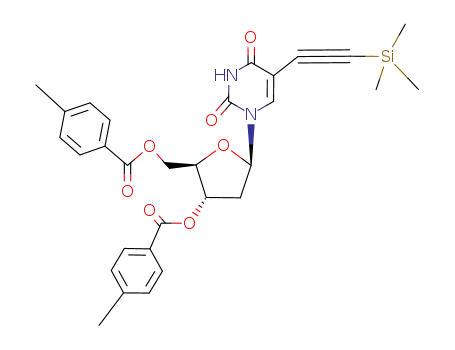

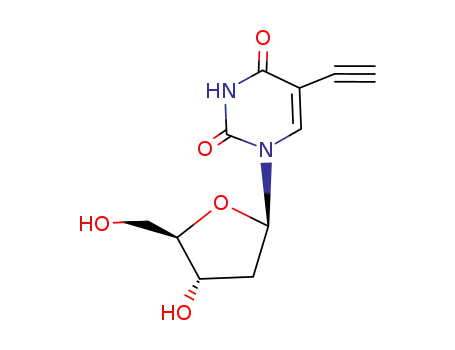
Conditions
Yield
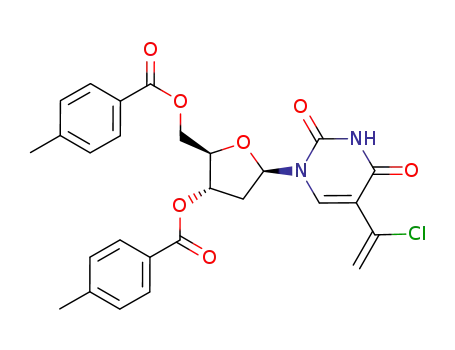

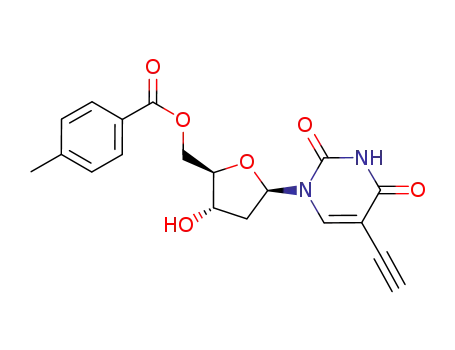


Conditions
Yield
12%
47%
61135-33-9 Upstream products


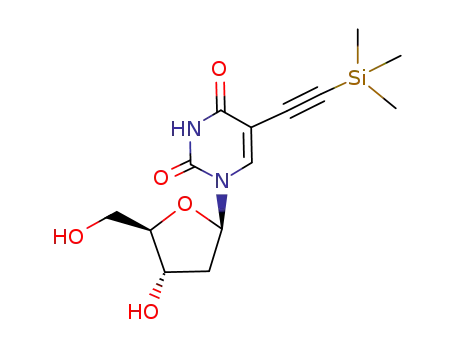
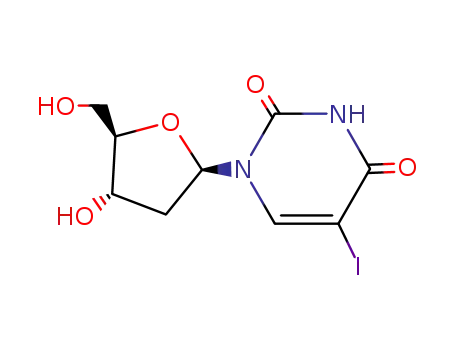
61135-33-9 Downstream products