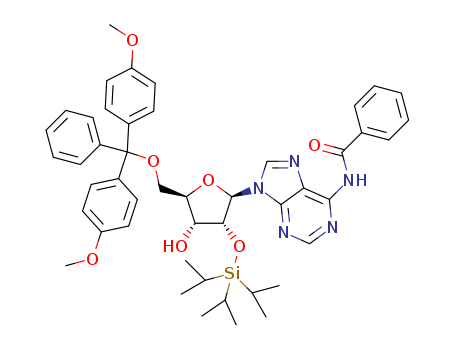
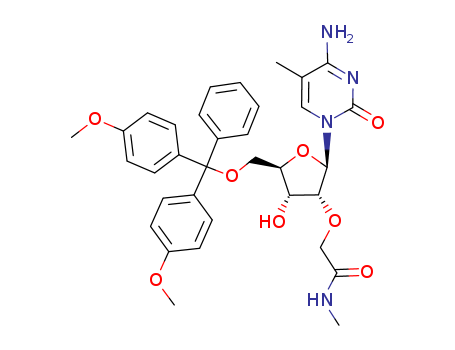
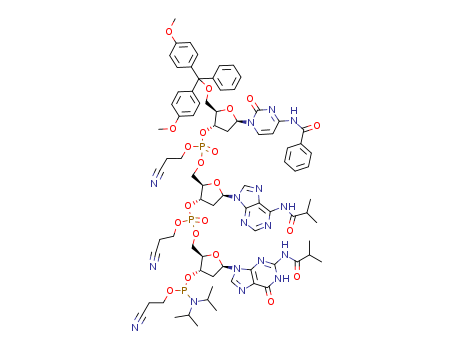
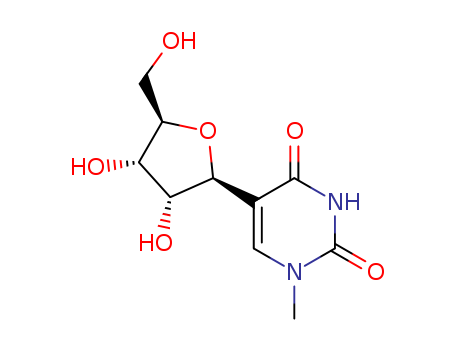
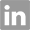Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
2004-07-1
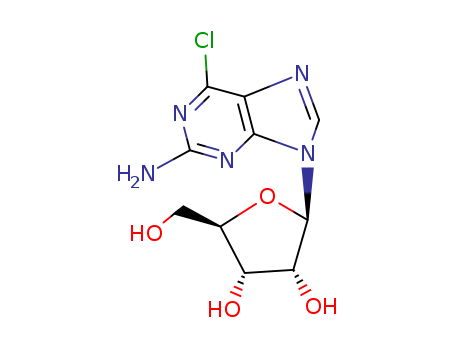
- Product Name:2-Amino-6-chloropurine-9-riboside
- Molecular Formula:C10H12ClN5O4
- Purity:99%
- Molecular Weight:301.689
Product Details;
CasNo: 2004-07-1
Molecular Formula: C10H12ClN5O4
Appearance: colourless crystalline solid
Chemical Properties Colourless Crystalline Solid Uses 6-Substituted purines; a novel class of inhibitors of endogenous protein degradation InChI:InChI=1/C10H12ClN5O4/c11-7-4-8(15-10(12)14-7)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)
O6-Methylguanosine derivative was treate...
-
The invention relates to the technical f...
CD73 inhibitors are promising drugs for ...
New nucleoside derivatives with nitrogen...
We have previously developed a new class...
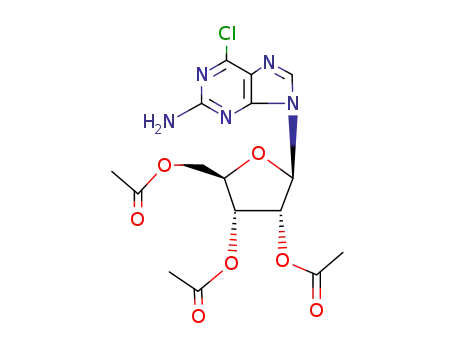
7-methylguanosine hydroiodide 2-Amino-6-chloropurin 2-Amino-6-chloropurine riboside 7-methylguanine 2-amino-6-chloro-9-(2,3,5-tri-O-acetyl-β-D-ribofuranosyl)purine 2-Amino-6-chloropurine riboside
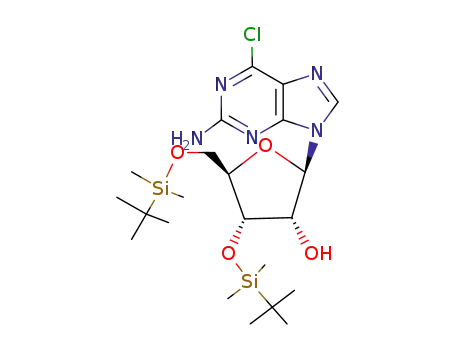
2-amino-6-chloro-9-<3,5-bis-O-(tert-butyldimethylsilyl)-β-D-ribofuranosyl>purine
2-amino-6-chloro-9-(2,3,5-tri-O-acetyl-β-D-ribofuranosyl)purine
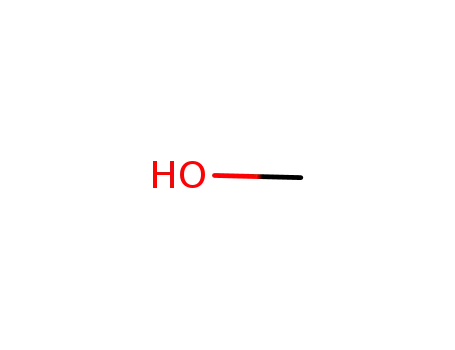
methanol
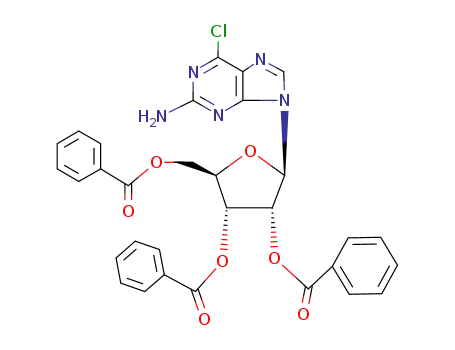
2-amino-6-chloro-9-(2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)purine
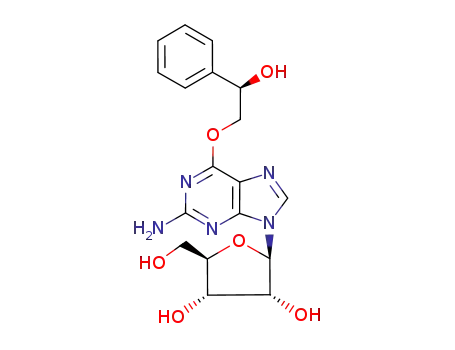
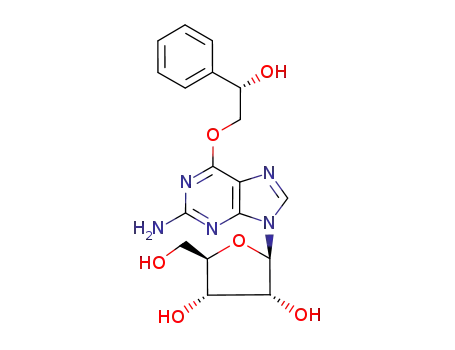
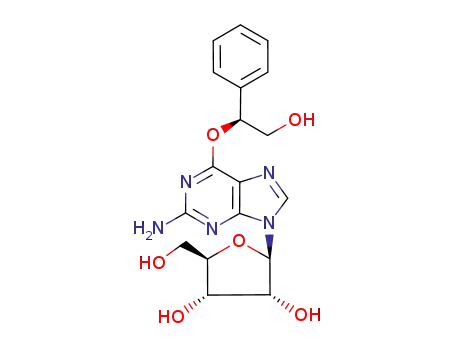
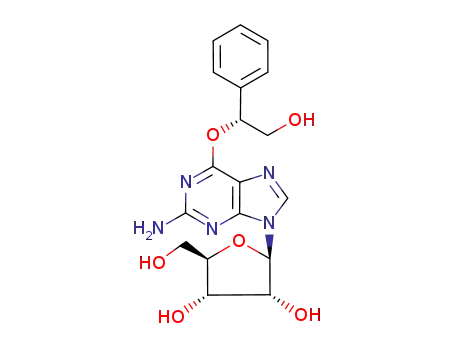
O6-(2-hydroxy-2-phenylethyl)guanosine
O6-(2-hydroxy-2-phenylethyl)guanosine
O6-(2-hydroxy-1-phenylethyl)guanosine
O6-(2-hydroxy-12-phenylethyl)guanosine
Reasoble Price High Purity 2-Amino-6-chloropurine-9-riboside 2004-07-1 Good Producer
2-Amino-6-chloropurine-9-riboside(Cas 2004-07-1) Usage
2004-07-1 Relevant articles
Reaction of O6-methylguanosine with nitrite in the presence of carboxylic acid: Synthesis of the purin-2-yl carboxylate
Maruyama, Tokumi,Moriwaka, Nobuyasu,Demizu, Yosuke,Ohtsuka, Masami
, p. 8225 - 8228 (2005)
-
Kikugawa,K. et al.
, p. 387 - 390 (1972)
Intermediate for synthesizing 2-chloroadenosine, synthesis process of intermediate and synthesis process of 2-chloroadenosine
-
Paragraph 0051-0052; 0057-0059; 0060-0068, (2021/01/25)
2-Substituted α,β-Methylene-ADP Derivatives: Potent Competitive Ecto-5′-nucleotidase (CD73) Inhibitors with Variable Binding Modes
Bhattarai, Sanjay,Pippel, Jan,Scaletti, Emma,Idris, Riham,Freundlieb, Marianne,Rolshoven, Georg,Renn, Christian,Lee, Sang-Yong,Abdelrahman, Aliaa,Zimmermann, Herbert,El-Tayeb, Ali,Müller, Christa E.,Str?ter, Norbert
supporting information, p. 2941 - 2957 (2020/04/10)
Synthesis of novel 6-substituted amino-9-(β-D-ribofuranosyl)purine analogs and their bioactivities on human epithelial cancer cells
Tuncbilek, Meral,Kucukdumlu, Asl?gul,Guven, Ebru Bilget,Altiparmak, Duygu,Cetin-Atalay, Rengul
, p. 235 - 239 (2018/02/15)
Base-modified GDP-mannose derivatives and their substrate activity towards a yeast mannosyltransferase
Collier, Alice,Wagner, Gerd K.
, p. 91 - 96 (2017/10/30)
2004-07-1 Process route
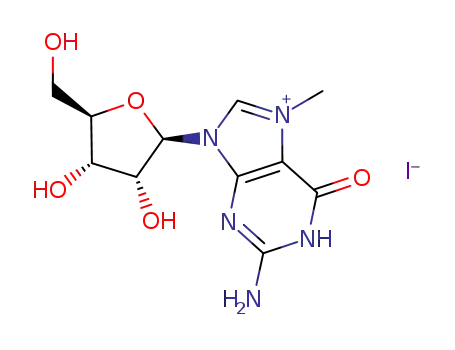

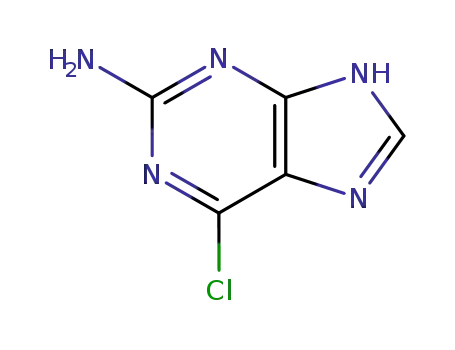

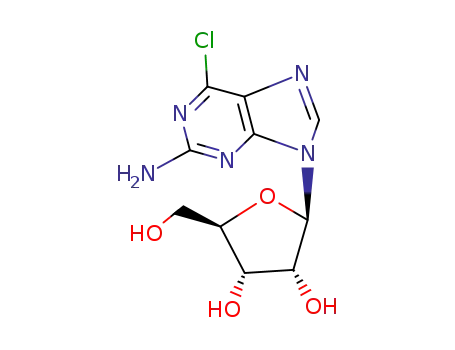

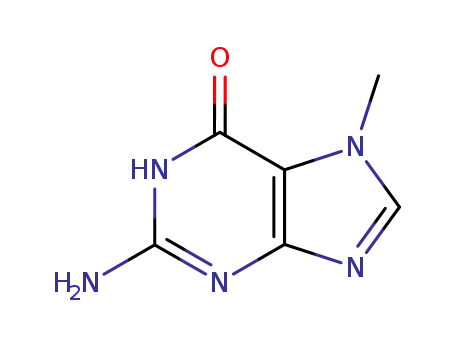
Conditions
Yield
93%


Conditions
Yield
93%
80%
55%
2004-07-1 Upstream products

2004-07-1 Downstream products