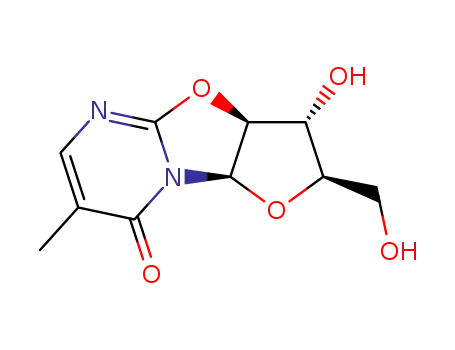
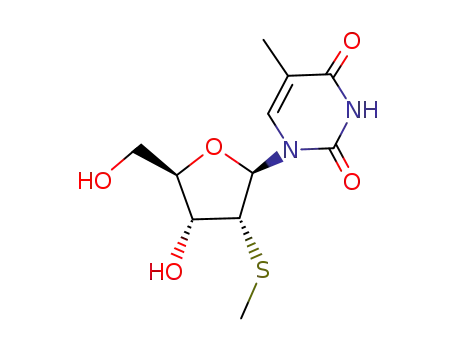
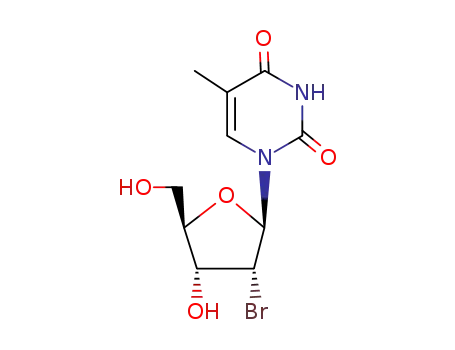
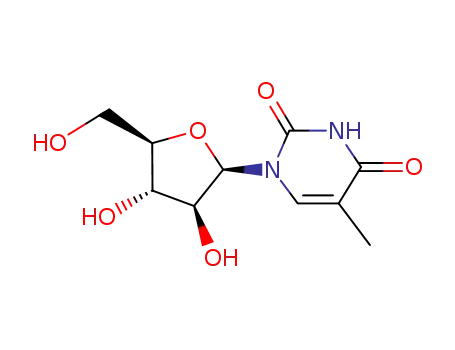
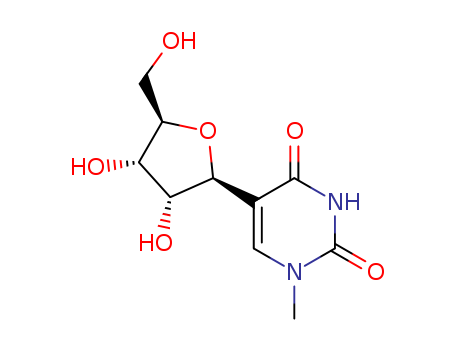
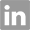Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
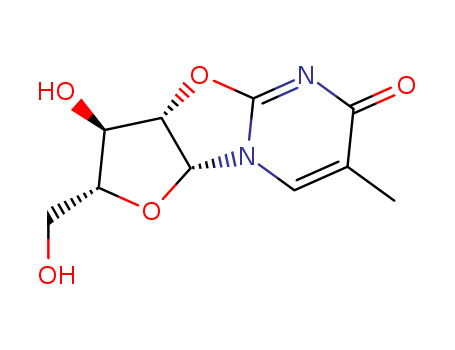
22423-26-3
- Product Name:2,2'-Anhydro-5-methyluridine
- Molecular Formula:C10H12N2O5
- Purity:99%
- Molecular Weight:240.216
Product Details;
CasNo: 22423-26-3
Molecular Formula: C10H12N2O5
Appearance: Colourless solid
Arabinoaminooxazoline reacted readily wi...
The invention relates to a preparation m...
Provided herein, for example, are method...
We describe herein a straightforward and...
A small library of thirty-two 2'-triazol...
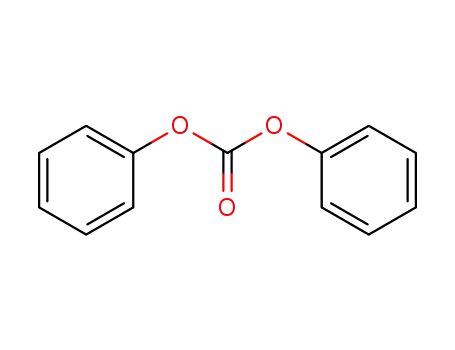
5-Methyluridine O-2,2'-cyclo-5-methyluridine bis(phenyl) carbonate 5-Methyluridine O-2,2'-cyclo-5-methyluridine
5-Methyluridine
N,N-dimethyl-formamide
1-(β-D-xylofuranosyl)-thymine
1-(3,5-O-sulfinyl-β-D-xylofuranosyl)thymine
(3aS)-3c-hydroxy-2t-hydroxymethyl-7-methyl-(3ar,9ac)-2,3,3a,9a-tetrahydro-furo[2',3':4,5]oxazolo[3,2-a]pyrimidin-8-one
2'-deoxy-2'-methylthio-5-methyluridine
2'-bromothymidine
thymine arabinoside
Reliable Quality Factory Sells For Sale 2,2'-Anhydro-5-methyluridine 22423-26-3
22423-26-3 Relevant articles
Facile Synthesis of 5-Substituted Arabinofuranosyluracil Derivatives
Sawai, Hiroaki,Hayashi, Hidekazu,Sekiguchi, Sumie
, p. 605 - 606 (1994)
Method for preparing β-thymidine
-
Paragraph 0096-0098; 0110, (2018/02/04)
METHODS FOR THE PREVENTION AND TREATMENT OF MAJOR ADVERSE CARDIOVASCULAR EVENTS USING COMPOUNDS THAT MODULATE APOLIPOPROTEIN B
-
Paragraph 00639, (2016/03/22)
A new straightforward synthesis of 2′,3′-didehydro-2′,3′-dideoxy-2′-(2″-(trimethylsilyl)ethylthio)thymidine, key intermediate for the synthesis of 2′-substituted thionucleosides
Oliveira, Maralise P.,Franco, Lucas L.,Lima, Maria C.A.,Sim?es, Cláudia M.O.,Galdino, Suely L.,Pitta, Ivan R.,Décout, Jean-Luc,Alves, Ricardo J.
, p. 816 - 821 (2015/04/14)
Cu(I)-catalyzed efficient synthesis of 2'-triazolo-nucleoside conjugates
Mathur,Rana,Olsen,Parmar,Prasad
, p. 701 - 710 (2015/05/13)
22423-26-3 Process route
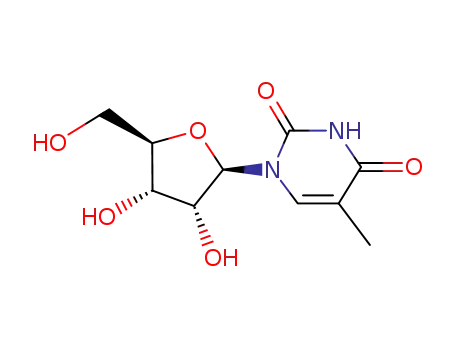

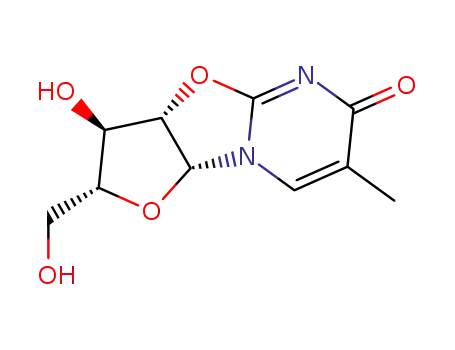
Conditions
Yield
95.6%
90%
85%
78%
64%
62%
47%




Conditions
Yield
85%
22423-26-3 Upstream products

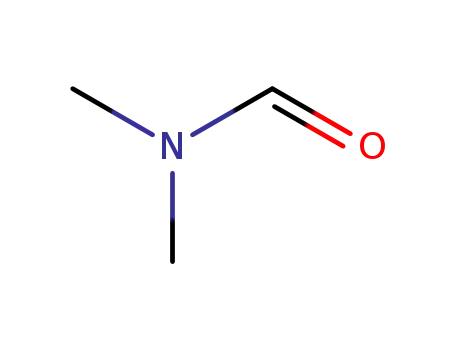
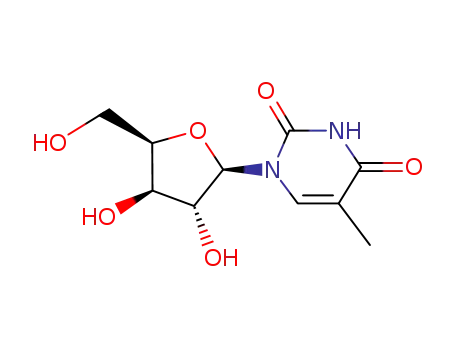
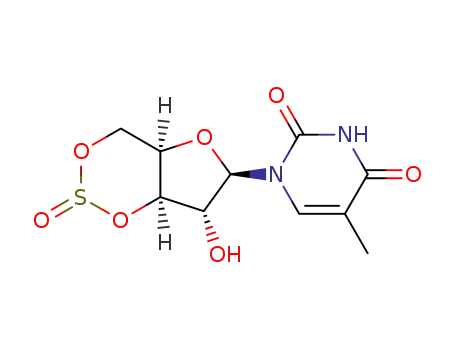
22423-26-3 Downstream products