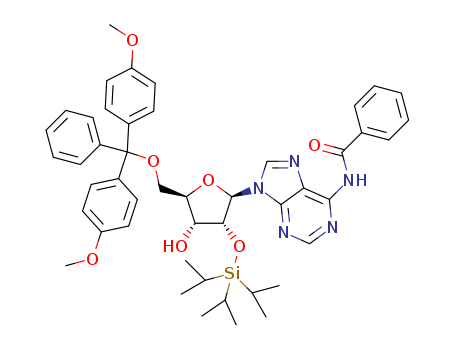
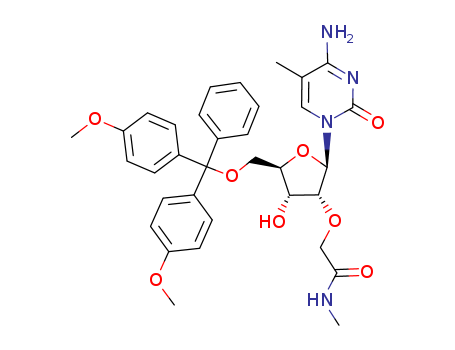
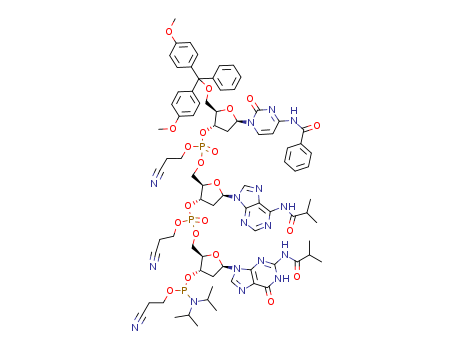
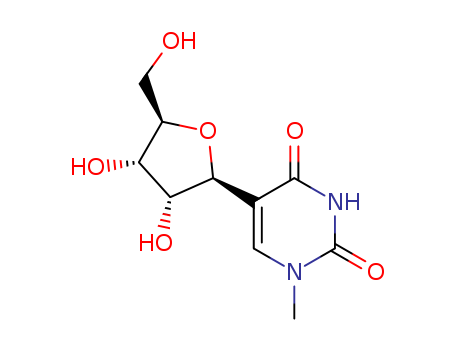
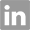Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
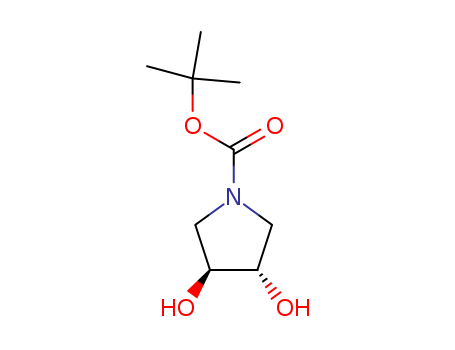
90481-33-7
- Product Name:N-BOC-(3S,4S)-3,4-PYRROLIDINEDIOL
- Molecular Formula:C9H17NO4
- Purity:99%
- Molecular Weight:203.238
Product Details;
CasNo: 90481-33-7
Molecular Formula: C9H17NO4
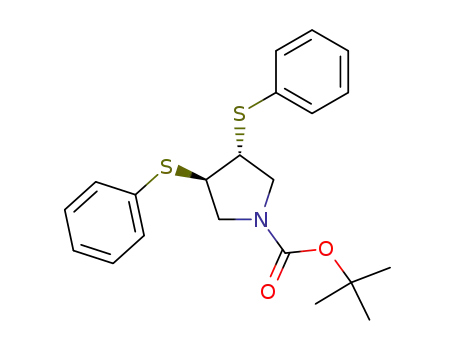
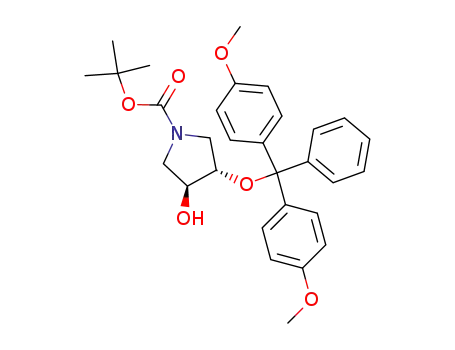
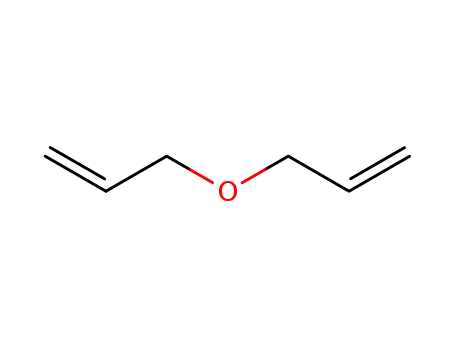
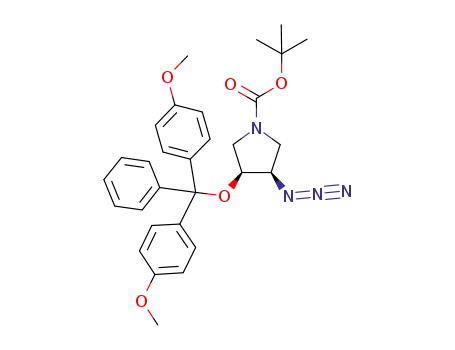
N-BOC-(3S,4S)-3,4-Pyrrolidinediol is a synthetic compound that has been developed for use as a chiral building block in organic synthesis. It is a derivative of pyrrolidine, a five-membered nitrogen-containing heterocycle that is commonly found in natural products and pharmaceuticals. The N-BOC (tert-butyloxycarbonyl) protecting group is used to prevent unwanted reactions during chemical synthesis, allowing for selective modification of the pyrrolidine ring. The (3S,4S) stereochemistry refers to the orientation of the two substituents on the pyrrolidine ring, which can have a significant impact on the properties and activity of the final product. N-BOC-(3S,4S)-3,4-Pyrrolidinediol is widely used in the pharmaceutical industry for the synthesis of a variety of biologically active compounds, including antiviral agents, antibiotics, and anticancer drugs. InChI:InChI=1/C9H17NO4/c1-9(2,3)14-8(13)10-4-6(11)7(12)5-10/h6-7,11-12H,4-5H2,1-3H3/t6-,7-/m0/s1 Traditional approaches to stereoselectiv... The invention discloses a compound which... The invention discloses compounds which ... Disclosed are a compound of Formula I ha... di-tert-butyl dicarbonate (3S,4S)-pyrrolidine-3,4-diol S,S-3,4-dihydroxypyrrolidine-1-carboxylic acid tert-butyl ester 1-tert-butoxycarbonyl-3,4-epoxypyrrolidine S,S-3,4-dihydroxypyrrolidine-1-carboxylic acid tert-butyl ester di-tert-butyl dicarbonate (3S,4S)-pyrrolidine-3,4-diol (3S,4S)-(+)-1-benzyl-3,4-pyrrolidinediol tert-butyl formate (+)-(3R,4R)-1-tert-butoxycarbonyl-3,4-bis(phenylsulfanyl)pyrrolidine (3S,4S)-1-N-tert-butyloxycarbonyl-3-(4,4'-dimethoxytrityloxy)-4-hydroxypyrrolidine Allyl ether (3R,4S)-tert-butyl 3-azido-4-(bis(4-methoxyphenyl)(phenyl)methoxy)pyrrolidine-1-carboxylateHigh Purity Low Price N-BOC-(3S,4S)-3,4-PYRROLIDINEDIOL 90481-33-7
N-BOC-(3S,4S)-3,4-PYRROLIDINEDIOL(Cas 90481-33-7) Usage
90481-33-7 Relevant articles
Selective Isomerization via Transient Thermodynamic Control: Dynamic Epimerization of trans to cis Diols
Macmillan, David W. C.,Oswood, Christian J.
supporting information, p. 93 - 98 (2022/01/03)
Compound JK-03M having higher protein kinase G inhibitory activity or pharmaceutically acceptable salt thereof and preparation method thereof
-
Paragraph 0093; 0118; 0135-0137, (2018/11/03)
Compounds with higher PKG (protein kinase G) inhibitory activity and preparation method of compounds
-
Paragraph 0160; 0161; 0162; 0163; 0164; 0165, (2016/10/08)
COMPOUND HAVING HIGHER INHIBITION OF PROTEIN KINASE G ACTIVITY AND PREPARATION METHOD THEREFOR
-
Paragraph 0075; 0076, (2016/12/12)
90481-33-7 Process route
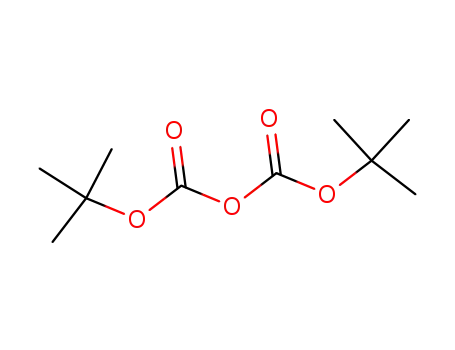

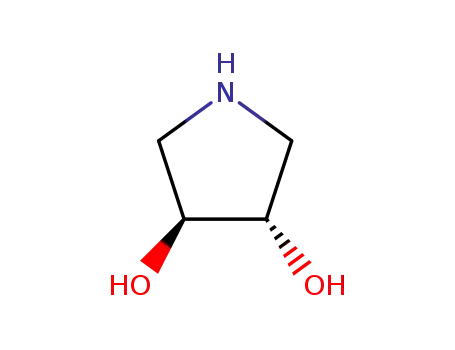

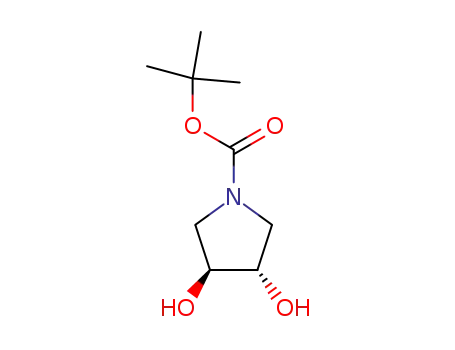
Conditions
Yield
86%
85%
84.6%
84.6%
84.6%
75%
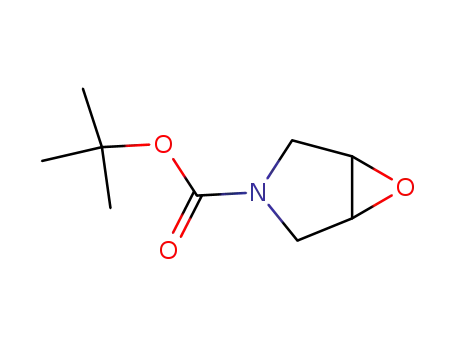


Conditions
Yield
91%
90481-33-7 Upstream products


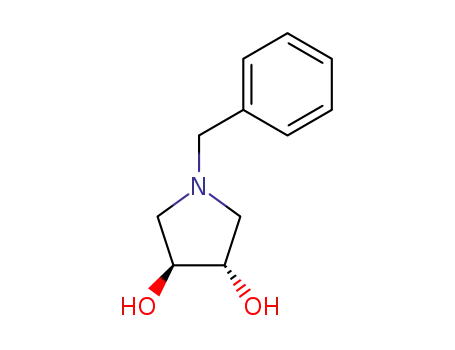
90481-33-7 Downstream products