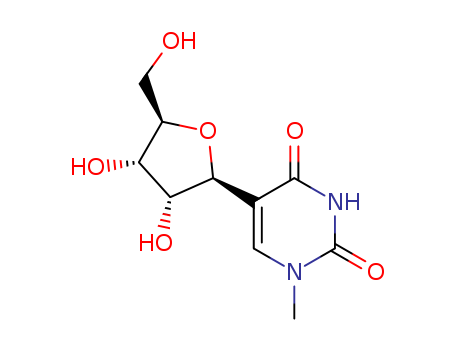
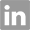Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
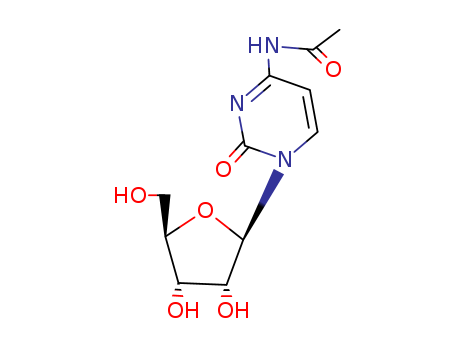
3768-18-1
- Product Name:N4-Acetylcytidine
- Molecular Formula:C11H15N3O6
- Purity:99%
- Molecular Weight:285.257
Product Details;
CasNo: 3768-18-1
Molecular Formula: C11H15N3O6
Appearance: white to off-white crystalline powder
Description N4-Acetylcytidine is a modified nucleoside and endogenous urinary nucleoside product of the degradation of tRNA. N4-Acetylcytidine is a biological marker for various cancers with elevated concentrations present in urine. tRNA has been shown to be excreted in abnormal amounts in the urine of cancer patients. tRNA from neoplastic tissue had a much more rapid turnover rate than the tRNA from the corresponding normal tissue. Evidence indicates that methylation of tRNA occurs only after synthesis of the intact macromolecule. Because there are no specific enzyme systems to incorporate the modified nucleosides into the macromolecular nucleic acid, these nucleosides once released in the process of tRNA turnover cannot be reutilized, nor are they further degraded, but are excreted in urine.N4-Acetylcytidine is also a partially protected cytidine and therefore can be used as a synthetic building block to prepare further derivatized nucleosides such as 2’,3’-dideoxycytidine. Uses N4-Acetylcytidine (cas# 3768-18-1) is a nucleotide-derived metabolite used as biomarkers for diagnosis of inflammation-related diseases. Definition ChEBI: N(4)-acetylcytidine is cytidine in which one of the exocyclic amino hydrogens is substituted by an acetyl group. It has a role as a metabolite. It is a member of cytidines, a member of acetamides and a secondary carboxamide. Biosynthesis N4-acetylcytidine (ac4C) is a post-transcriptional modification of RNA that is conserved across all domains of life. All characterized sites of ac4C in eukaryotic RNA occur in the central nucleotide of a 5’-CCG-3’ consensus sequence. However, the thermodynamic consequences of cytidine acetylation in this context have never been assessed due to its challenging synthesis. Here we report the synthesis and biophysical characterization of ac4C in its endogenous eukaryotic sequence context. First, we develop a synthetic route to homogenous RNAs containing electrophilic acetyl groups. Next, we use thermal denaturation to interrogate the effects of ac4C on duplex stability and mismatch discrimination in a native sequence found in human ribosomal RNA. Finally, we demonstrate the ability of this chemistry to incorporate ac4C into the complex modification landscape of human tRNA, and use duplex melting combined with sequence analysis to highlight a potentially unique enforcing role for ac4C in this setting. By enabling the analysis of nucleic acid acetylation in its physiological sequence context, these studies establish a chemical foundation for understanding the function of a universally-conserved nucleobase in biology and disease.Site-Specific Synthesis of N4-Acetylcytidine in RNA Reveals Physiological Duplex Stabilizationhttps://www.biorxiv.org/content/10.1101/2021.11.12.468326v1.full.pdf InChI:InChI=1/C11H15N3O6/c1-5(16)12-7-2-3-14(11(19)13-7)10-9(18)8(17)6(4-15)20-10/h2-3,6,8-10,15,17-18H,4H2,1H3,(H,12,13,16,19)/t6-,8-,9-,10-/m1/s1
A convenient synthesis of the title comp...
-
Phosphonate derivatives of cytidine and ...
N4-Acetylcytidine (77%) and 2′,3′-O, N4-...
Both the (R)- and (S)-5′-hydroxy 5′-phos...
The invention relates to the field of ph...
Disclosed herein are nucleotide analogs ...
-
The use of an acetylated benzotriazole f...
acetic anhydride CYTIDINE N-acetylcytidine CYTIDINE N-acetylcytidine
acetyl chloride
O2',O3',O5'-tris-trimethylsilanyl-cytidine
acetic anhydride
CYTIDINE
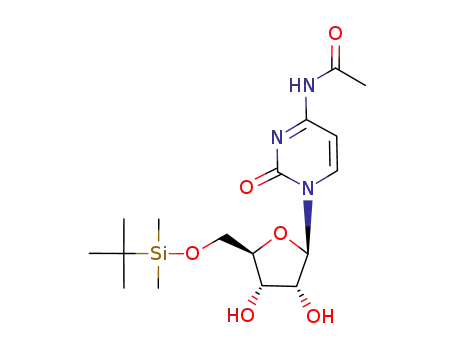
N4-acetyl-5'-O-(tert-butyldimethylsilyl)cytidine
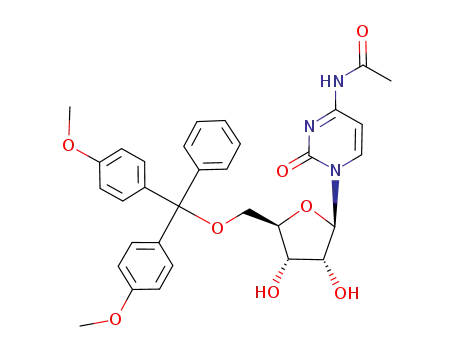
N4-acetyl-5'-O-(4,4'-dimethoxytrityl)cytidine
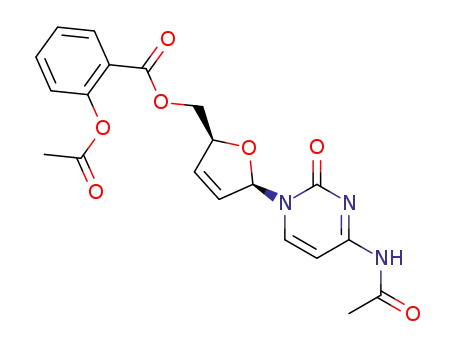
2-Acetoxy-benzoic acid (2S,5R)-5-(4-acetylamino-2-oxo-2H-pyrimidin-1-yl)-2,5-dihydro-furan-2-ylmethyl ester
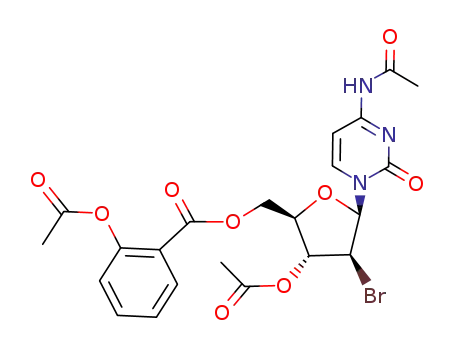
2-Acetoxy-benzoic acid (2R,3R,4S,5R)-3-acetoxy-5-(4-acetylamino-2-oxo-2H-pyrimidin-1-yl)-4-bromo-tetrahydro-furan-2-ylmethyl ester
High Purity Low Price Hot Sale N4-Acetylcytidine 3768-18-1
N4-Acetylcytidine(Cas 3768-18-1) Usage
3768-18-1 Relevant articles
A convenient synthesis of N4,O3′,O 5′-triacetyl-2′-ketocytidine
Kjell, Douglas P.,Slattery, Brian J.
, p. 469 - 474 (1997)
-
Ohashi et al.
, p. 209,211, 212 (1972)
Phosphonate analogues of cytosine arabinoside monophosphate
Chen, Xuemei,Jung, Kang-Yeoun,Wiemer, David F.,Wiemer, Andrew J.,Hohl, Raymond J.
, p. 1783 - 1786 (2002)
Synthesis of regioselectively protected forms of cytidine based on enzyme-catalyzed deacetylation as the key step
Kuboki, Atsuhito,Ishihara, Takashi,Kobayashi, Eiko,Ohta, Hiromichi,Ishii, Takeshi,Inoue, Ayumu,Mitsuda, Satoshi,Miyazaki, Tatsuo,Kajihara, Yasuhiro,Sugai, Takeshi
, p. 363 - 368 (2000)
Stereoselective synthesis of the 5′-hydroxy-5′-phosphonate derivatives of cytidine and cytosine arabinoside
Chen, Xuemei,Wiemer, Andrew J.,Hohl, Raymond J.,Wiemer, David F.
, p. 9331 - 9339 (2002)
Preparation method of 3'-deoxyuridine
-
Paragraph 0033; 0034; 0035; 0036, (2017/09/02)
SUBSTITUTED NUCLEOSIDE AND NUCLEOTIDE ANALOGS
-
Page/Page column 138, (2010/10/03)
An efficient one-pot N-acylation of deoxy- and ribo-cytidine using carboxylic acids activated in situ with 2-chloro-4,6-dimethoxy-1,3,5-triazine
Rode, Ambadas B.,Son, Sang Jun,Hong, In Seok
experimental part, p. 2061 - 2064 (2010/11/19)
Electron-deficient benzotriazoles for the selective N-acetylation of nucleosides
Reid, Andrew K.,McHugh, Callum J.,Richie, Graham,Graham, Duncan
, p. 4201 - 4203 (2007/10/03)
3768-18-1 Process route
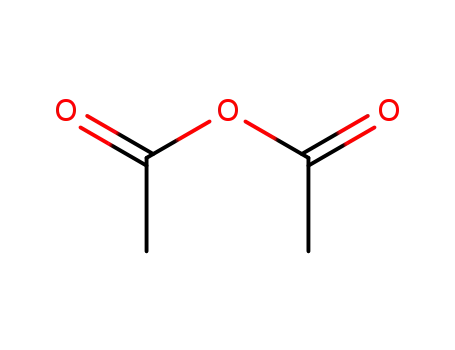

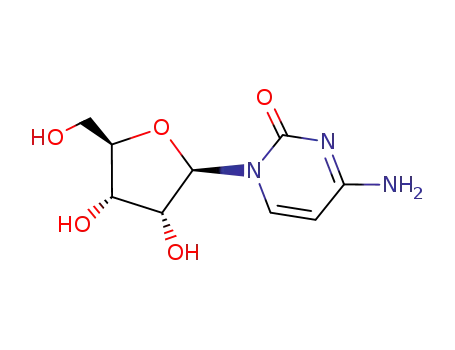

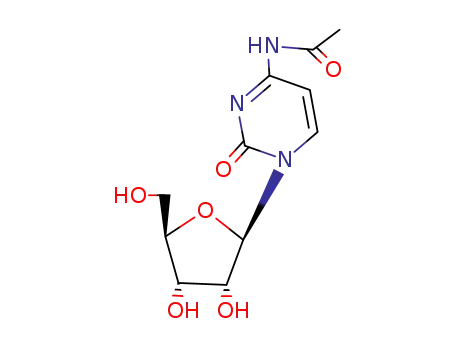
Conditions
Yield
96%
96%
95%
89%
87%
84%



Conditions
Yield
100%
3768-18-1 Upstream products
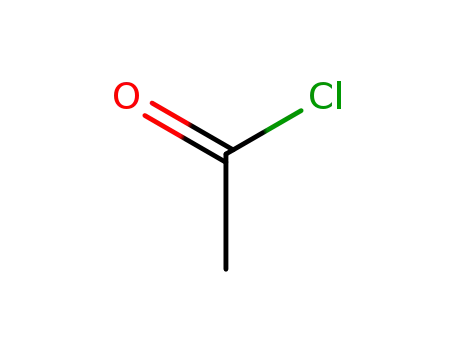
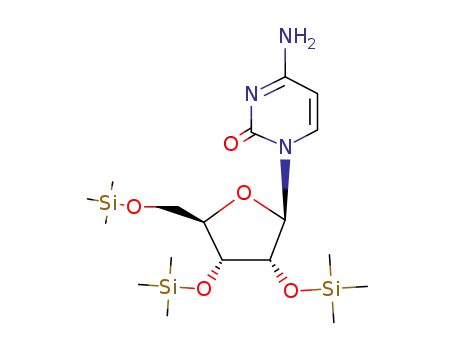


3768-18-1 Downstream products