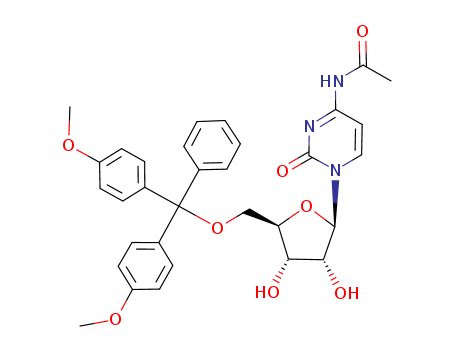
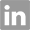Product Classification
Contact us
Tel:021-6710 8196
Phone:191 2194 8821
Website:https://www.xhmedi.com
Email: sales@xhmedic.com
Address:Room 306, Building A, 1888 Wangyuan Road, Fengxian District, Shanghai,China
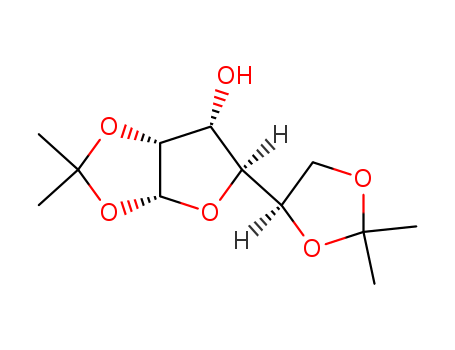
2595-05-3
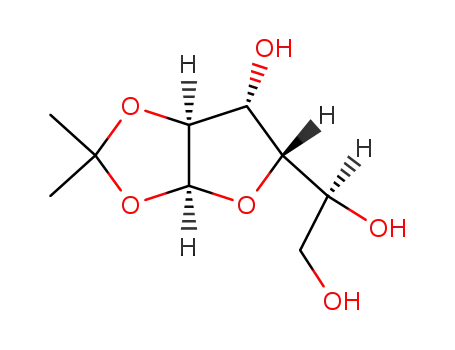
- Product Name:1,2:5,6-Di-O-isopropylidene-alpha-D-allofuranose
- Molecular Formula:C7H9N3O2
- Purity:99%
- Molecular Weight:260.287
Product Details;
CasNo: 2595-05-3
Molecular Formula: C7H9N3O2
Synthesis Anhydrous DMSO (650 mL) was cooled to 18?20 °C under nitrogen in a 3-L roundbottomed glass flflask. DMSO solidififies at 18 °C and therefore it is important to keep the reaction mixture just above freezing point. To this cold solution was added P2O5 (142 g, 1.0 mol, 1 equivalent) in 3 portions under a N2 atmosphere. The addition of P2O5?to DMSO is exothermic, and if the mass temperature exceeds 28 °C, the color darkens and the product will be of inferior quality. The mixture was cooled to 18?20 °C between each addition. After addition of P2O5 was completed, the mixture was stirred at 18?25 °C for 10?15 min. 1,2:5,6-Di-O-isopropylidene-D-glucofuranose (260 g, 1.0 mol) was dissolved in anhydrous DMSO (1.3 L) and added over 30 min (maintaining the temperature at 18?25 °C) to the stirred solution of P2O5 in DMSO under a N2 atmosphere. The resulting solution was heated to 50?55 °C for 3 h. TLC (eluent: CH2Cl2:MeOH, 95:5) shows complete conversion of glucofuranose (Rf = 0.68) to ulose (Rf = 0.81). The reaction mixture was allowed to reach 25?30 °C and was extracted twice with methyl tert-butyl ether (MTBE 1.5 and 1 L) in a 6-L separation funnel. The combined MTBE layer (~4 L) was concentrated in vacuo (water-bath temperature set to 40 °C) to approximately 2 L and allowed to reach 25?30 °C. NaBH4 (24 g, 0.63 mol) was dissolved in water (1 L, 55.6 mol) at 0?10 °C, and the concentrated MTBE layer was added to the aqueous layer over 30 min to keep ?the temperature at 0?10 °C. TLC (eluent: EtOAc/heptane, 6:4) after 30 min shows ?full conversion of ulose (Rf = 0.53) to 1,2:5,6-di-O-isopropylidene-D-allofuranose (Rf = 0.39). The reaction mixture was allowed to reach 25?30 °C. CH2Cl2 (1 L) and water (500 mL) were added, and the layers were separated. The aqueous layer was extracted once more with CH2Cl2 (500 mL). The combined organic layers were concentrated in vacuo to an oil which was subsequently dissolved in MTBE (300 mL) and extracted with water (3 × 500 mL). The combined aqueous layers were extracted with CH2Cl2 (3 × 500 mL). The combined CH2Cl2 layers were dried (Na2SO4, 100 g), fifiltered, and concentrated in vacuo to provide the crude oil. Crystallization from cyclohexane (500 mL), washing of crystals with cold n-pentane, and drying hereof in vacuo afforded analytically pure 1,2:5,6-di-O-isopropylidene-D-allofuranose (191 g, 73%). Reference: Christensen, S. M.; Hansen, H. F.; Koch, T. Org. Proc. Res. Dev. 2004, 8, 777?780. Chemical Properties White Solid Uses Protected α-D-Allofuranose InChI:InChI=1/C12H20O6/c1-11(2)14-5-6(16-11)8-7(13)9-10(15-8)18-12(3,4)17-9/h6-10,13H,5H2,1-4H3/t6-,7-,8-,9-,10-/m1/s1
A series of novel 5-((1H-benzo[d]imidazo...
A series of novel 5-((3aR,5S,6S,6aR)-6-(...
A highly selective, mild, and efficient ...
AprD4 is a radical S-adenosyl-l-methioni...
The present invention relates to TLR7 ag...
The present invention relates to a gluco...
3-O-acetyl-1,2,5,6-di-isopropylidene-α-D-glucofuranose 1,2:5,6-Di-O-isopropylidene-α-D-glucofuranose acetic acid methyl ester D-altrose acetone 1,2:3,4-di-O-isopropylidene-β-D-altropyranose 1,2:5,6-Di-O-isopropylidene-β-D-altrofuranose
L-glucose
acetone
1,2-O-isopropylidene-α-L-glucofuranose
(2S,3S,4S)-6-Nitro-hexane-1,2,3,4,5-pentaol
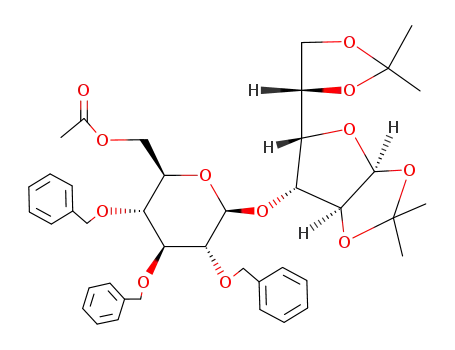
Acetic acid (2R,3R,4S,5R,6S)-3,4,5-tris-benzyloxy-6-[(3aS,5S,6R,6aS)-5-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl)-2,2-dimethyl-tetrahydro-furo[2,3-d][1,3]dioxol-6-yloxy]-tetrahydro-pyran-2-ylmethyl ester
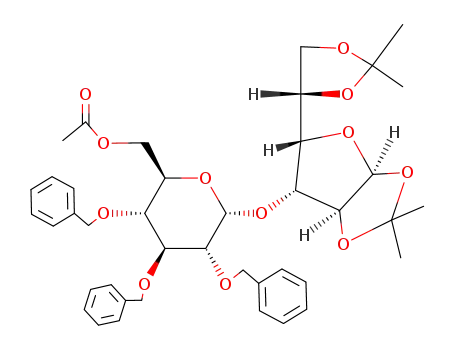
Acetic acid (2R,3R,4S,5R,6R)-3,4,5-tris-benzyloxy-6-[(3aS,5S,6R,6aS)-5-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl)-2,2-dimethyl-tetrahydro-furo[2,3-d][1,3]dioxol-6-yloxy]-tetrahydro-pyran-2-ylmethyl ester
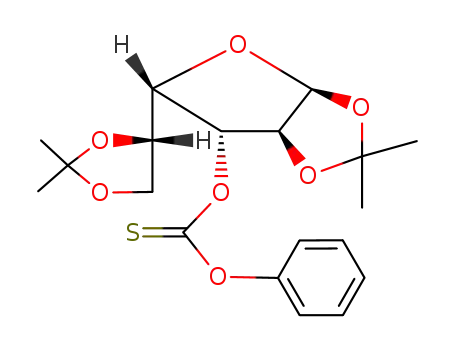
Thiocarbonic acid O-[(3aS,5S,6R,6aS)-5-((S)-2,2-dimethyl-[1,3]dioxolan-4-yl)-2,2-dimethyl-tetrahydro-furo[2,3-d][1,3]dioxol-6-yl] ester O-phenyl ester
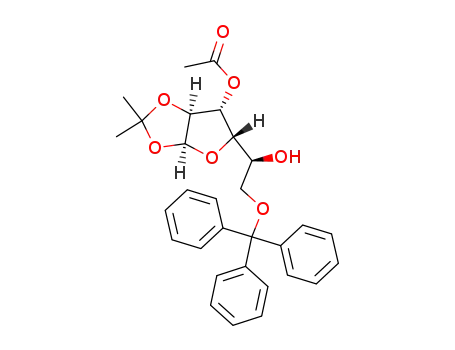
3-O-acetyl-1,2-O-isopropylidene-6-O-trityl-α-L-glucofuranose
Global Factory Sells 1,2:5,6-Di-O-isopropylidene-alpha-D-allofuranose 2595-05-3 In Stock
1,2:5,6-Di-O-isopropylidene-Alpha-D-Allofuranose(Cas 2595-05-3) Usage
2595-05-3 Relevant articles
Microwave-assisted Synthesis of Hybrid Heterocyclics as Biological Potent Molecules
Srinivas,Sunitha,Vasumathi Reddy,Karthik,Rajesh Kumar
, p. 1564 - 1573 (2018)
Synthesis and In Vitro Study of Hybrid Heterocyclic's as Potential Nematicidal Agents
Srinivas,Sunitha,Karthik,Nikitha,Raju,Ravinder,Anusha,Rajasri,Swapna,Swaroopa,Srinivas,Vasumathi Reddy
, p. 3250 - 3257 (2017)
Triethylamine-methanol mediated selective removal of oxophenylacetyl ester in saccharides
Rasool, Javeed Ur,Kumar, Atul,Ali, Asif,Ahmed, Qazi Naveed
, p. 338 - 347 (2021/01/29)
Mechanistic Investigation of 1,2-Diol Dehydration of Paromamine Catalyzed by the Radical S-Adenosyl- l -methionine Enzyme AprD4
Yeh, Yu-Cheng,Kim, Hak Joong,Liu, Hung-Wen
supporting information, p. 5038 - 5043 (2021/05/04)
TLR7 AGONISTS
-
, (2021/05/28)
GLUCOSIDE MONOMER, POLYMERIZATION COMPOSITION COMPRISING THE SAME AND HYDROGEL LENS USING THE SAME
-
Paragraph 0124-0130, (2019/08/22)
2595-05-3 Process route
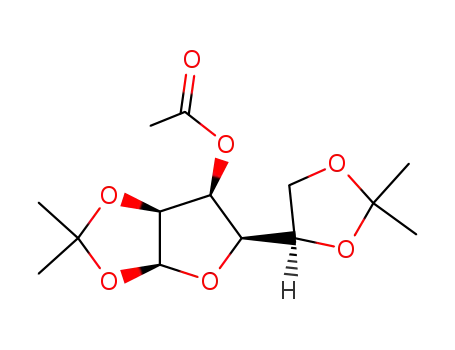

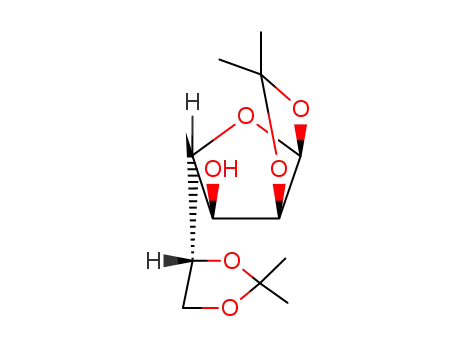

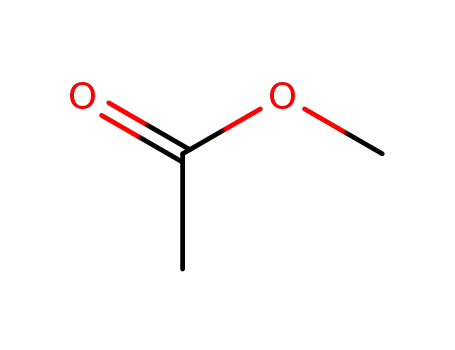
Conditions
Yield
100%
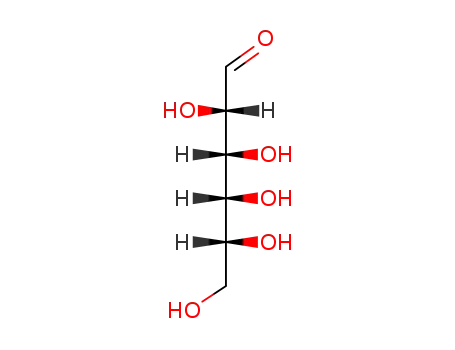

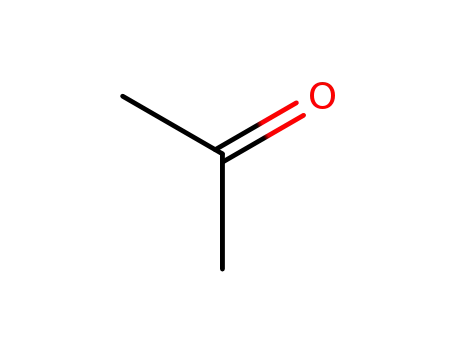

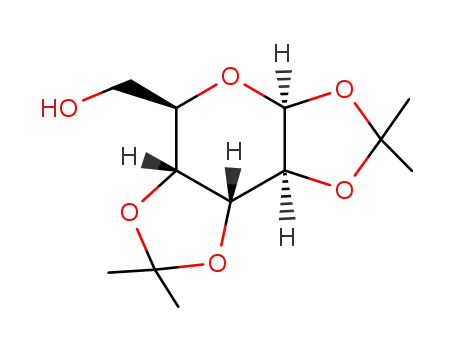

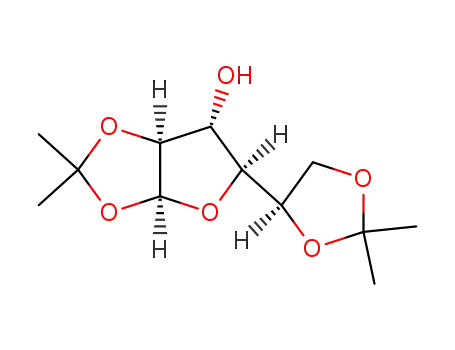
Conditions
Yield
2595-05-3 Upstream products
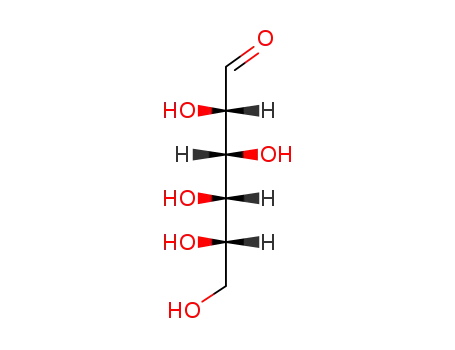

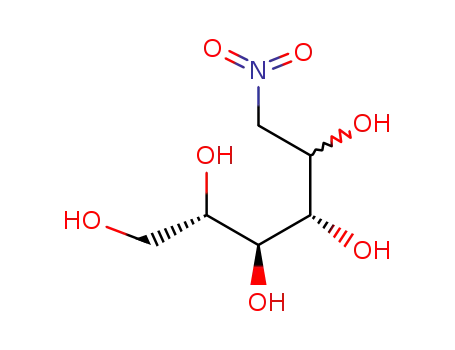
2595-05-3 Downstream products